Recent generations of continuous-flow left ventricular assist devices (CF-LVADs) offer improved survival and quality of life for end-stage heart failure (HF) patients.1–3 While initially used mainly as a bridge-to-transplant, more patients are undergoing LVAD implantation as destination therapy.1 With improving survival on LVAD support, the prevalence of LVAD-associated adverse events is increasing.1,3 Worsening aortic insufficiency (AI) is one such sequela of prolonged CF-LVAD support that has a significant impact on patient outcomes with well over 50% of patients developing mild disease at 2 years of support, and 15% developing moderate-to-severe disease.4–6 The primary mechanism proposed for the development of AI is an increase in the transvalvular gradient with simultaneous decompression of the left ventricle leading to aortic valve closure. Regurgitant flow can lead to reduced cardiac output, worsening heart failure symptoms and compromised end-organ perfusion.4,5 Patients with moderate-to-severe AI are at higher risk of rehospitalisations, haemocompatibility-related adverse events and mortality.6,7 International Society of Heart and Lung Transplantation guidelines recommend surgical treatment for more than mild AI at the time of LVAD implantation.8 However, appropriate management of post-implant AI remains a topic of contention.
Medical treatment, including diuretics, vasodilators and LVAD speed titration, may help to temporarily improve symptoms, but do not halt the progression of LVAD-associated AI.9 Despite these measures, some patients may continue to have clinically significant AI requiring additional intervention. Contemporary non-medical approaches to LVAD-associated AI include invasive surgery (including suture repair, aortic patch closure or surgical aortic valve replacement [SAVR]) and transcatheter interventions (including occluder devices and transcatheter aortic valve replacement [TAVR]).9–11 The data on patient outcomes with either SAVR or TAVR, however, are limited to small sample sizes from single-centre studies. We sought to analyse the National Inpatient Sample (NIS) database for trends and outcomes of LVAD patients who underwent TAVR or SAVR procedures for post-implant AI.
Methods
Sponsored by the Agency for Healthcare Research and Quality as part of the Healthcare Cost and Research Utilisation Project, the NIS database is the largest all-payer inpatient database in the US. The database is designed to produce regional and national estimates of inpatient usage, access, charges, quality and outcomes. In addition, it contains clinical and non-clinical data elements for each hospital stay, including, but not limited to, ICD-10, Clinical Modification/Procedure Coding System diagnosis, procedures codes, patient demographics, hospital characteristics, discharge status, length of stay, severity and comorbidity measures. The data include approximately 20% of stratified sample discharges from US hospitals; long-term acute care and rehabilitation hospitals are not included. A discharge weight variable is available to calculate the national estimates of the variables previously stated. Each record included in the NIS database is de-identified. We queried the NIS data collected for hospital admissions between the years 2015 and 2018 for all admissions of LVAD patients using ICD-10 and ICD-9 codes (Z95.811 and V43.21). Among those patients, we identified patients who underwent TAVR and SAVR using appropriate ICD-10 and CPT codes.
Our primary outcome was a composite of in-hospital mortality, stroke, transient ischaemic attack, MI, pacemaker implantation, need for open aortic valve surgery, vascular complications and cardiac tamponade. For the purposes of the SAVR cohort, the need for open aortic valve surgery was not included in the calculation of the primary outcome. Secondary outcomes included moderate-to-severe paravalvular regurgitation postprocedure, acute kidney injury and bleeding requiring transfusion.
Baseline characteristics were compared between patients undergoing TAVR and SAVR. To analyse the trends in the prevalence of TAVR and SAVR, we used both ICD-9 and ICD-10 codes, as the ICD-10 codes began to be used in October 2015. For the remainder of the analyses, we used ICD-10 codes to identify comorbidities and outcomes. Weighted data using the Healthcare Cost and Research Utilisation Project-recommended survey design were used to account for clustering and stratified sampling. The continuous variables were presented as median (interquartile range), and categorical variables were presented as proportions. The χ2 test was used to compare proportions, and the Wilcoxon rank-sum test was used to compare continuous variables. Univariate and multivariable logistic regression analyses were performed to assess the association between TAVR versus SAVR and outcomes. Given the limitations of sample size and event rate, no more than three independent variables were entered in the multivariable models to maintain model stability; the Elixhauser Comorbidity Index and non-elective nature of admission were selected a priori in the adjusted multivariable models.12 The rationale for choosing the Elixhauser Comorbidity Index and non-elective (versus elective) nature of admission in the adjusted model was to capture the burden of chronic comorbid conditions and acuity of illness, respectively. Statistical significance was met at p-value <0.05. All the statistical analyses were performed using SPSS 26.0 (IBM Corp) and Stata 13 (StataCorp LP) statistical packages.
Results
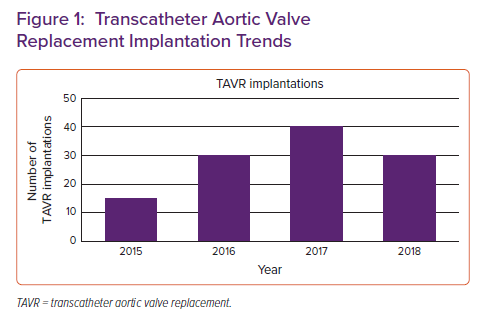
A total of 61,165 LVAD hospitalisations were identified during the study period; the number of LVAD hospitalisations increased steadily from 11,645 in the year 2015 to 19,470 in the year 2019. A total of 105 TAVR implantations and 50 SAVR procedures were performed in LVAD patients during the study period. As shown in Figure 1, there was an increase in the number of TAVR procedures performed annually during the study period, from 15 in 2015 to 30 in 2018. The small sample size limited our ability to report similar trends in SAVR.
Baseline Characteristics
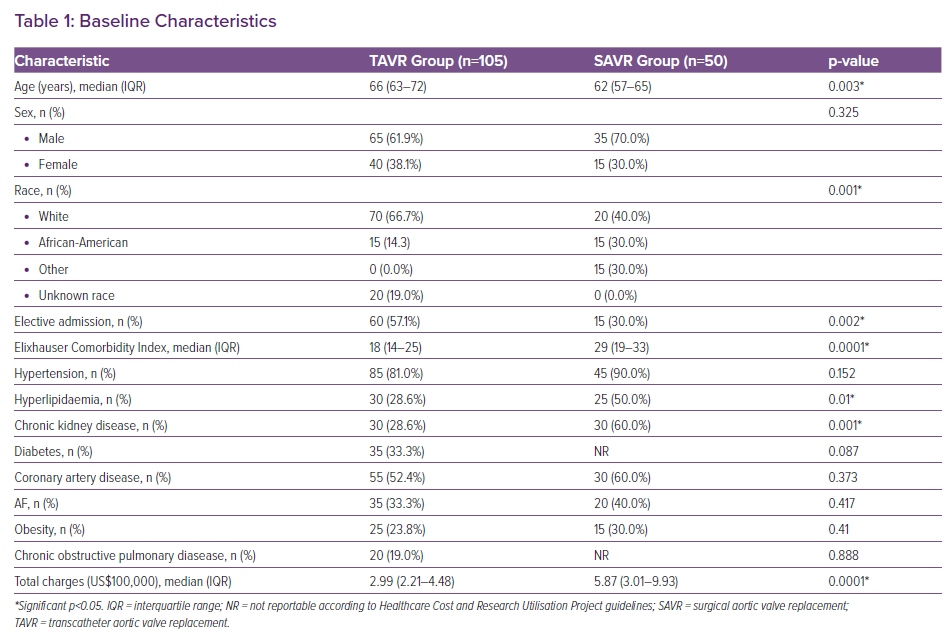
Overall, the median age of patients in the TAVR group was 66 years (interquartile range 63–72 years), 61.9% were male and 14.3% were African-American. Significant differences in baseline characteristics were observed between the TAVR and the SAVR groups (Table 1). Patients undergoing TAVR were more likely to undergo their procedure during an elective admission (57.1 versus 30%; p=0.002). A significantly higher prevalence of comorbidities, as assessed by the Elixhauser Comorbidity Index, was observed in the SAVR group (29 versus 18; p=0.0001). The median hospitalisation charge (multiples of US$100,000) was also higher in the SAVR group (5.87 versus 2.99; p=0.0001).
Outcomes with Transcatheter Aortic Valve Replacement and Surgical Aortic Valve Replacement
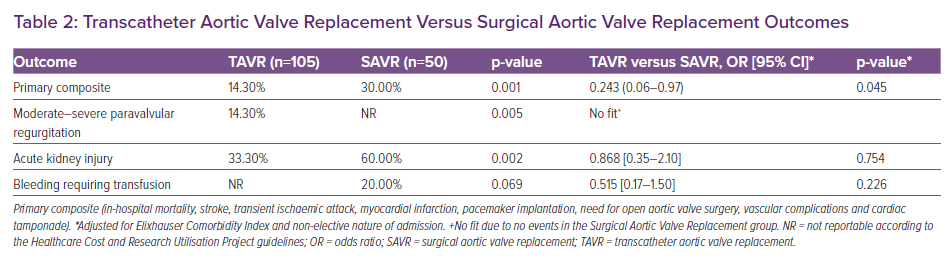
Table 2 outlines outcomes associated with TAVR and SAVR. We observed a significantly higher prevalence of the primary composite outcome in patients undergoing SAVR (30%) compared with those undergoing TAVR (14.3%; p=0.001). In the multivariable adjusted analyses, patients undergoing TAVR remained at significantly lower risk of the primary composite outcome compared with those undergoing SAVR (adjusted OR 0.24; 95% CI [0.06–0.97]; p=0.045).
Patients undergoing TAVR had a significantly higher prevalence of postprocedure moderate-to-severe paravalvular regurgitation; however, the difference was statically non-significant after adjustments in the multivariable model (Table 2). Similarly, while the prevalence of acute kidney injury and bleeding requiring transfusions were noted to be higher in the SAVR group, these differences did not retain statistical significance upon multivariable analyses (Table 2). Although the actual percentage of patients with paravalvular regurgitation in the SAVR group and bleeding requiring transfusion in the TAVR group were too low to report per the Healthcare Cost and Research Utilisation Project guidelines, the exact numbers were used to check for statistical significance.
Discussion
The development of post-implant AI is a known complication of prolonged CF-LVAD use, and is associated with increased morbidity and mortality in this cohort. As such, interest has grown in how best to manage post-implant AI in these patients.
In this national cohort, we observed that the number of patients undergoing TAVR for post-LVAD AI is increasing. Compared with the SAVR group, patients undergoing TAVR had a significantly lower burden of comorbidities and were more likely to undergo their procedure during an elective admission. It is indeed counter-intuitive that patients with a greater number of comorbidities were offered SAVR rather than TAVR. One possible explanation is that sicker hospitalised patients may have been deemed more suitable for an urgent surgical intervention rather than an elective TAVR procedure. Overall, we observed that the patients undergoing TAVR were at significantly less risk of poor outcomes when compared with the SAVR group.
A variety of surgical techniques have been attempted to address post-LVAD AI, including bioprosthetic valve replacement, valve repair and valve closure. In a single-centre case series of CF-LVAD patients with post-implant AI, Atkins et al. reported five patients undergoing surgical intervention (one treated with Dacron patch, two treated with bioprosthetic AVR and two treated with Park stitches), one of whom died perioperatively.10 In a separate cohort, Gyoten et al. reported six patients undergoing SAVR, four of whom needed temporary right ventricular assist devices, three of whom needed emergent heart transplantation and one of whom died perioperatively.13 In this national cohort of 50 patients undergoing SAVR in our study, we observed a similarly higher risk of in-hospital complications, including mortality in patients undergoing SAVR when compared with TAVR.
The TAVR procedure was initially indicated for use in patients with severe aortic stenosis who were high-risk surgical candidates.14 As comfort with the TAVR procedure has increased and technology has developed, indications for its use have expanded. Early use of TAVR as a treatment for isolated native valve AI was limited by anatomical and technical limitations, including difficulty anchoring the valve due to lack of calcium, leading to valve instability and an increased risk of valve malpositioning and paravalvular leakage.15–18 Recent developments in the ability to size the aortic annulus with pre-implantation CT, as well as recent advances in percutaneous bioprosthetic valve technology, has led to a renewed interest in the use of TAVR for treatment of AI.18,19 Newer-generation valves have additional advantages that contribute to the increased success in the use of TAVR for AI, including expandability, retrievability and repositioning capacity, and unique anchoring mechanisms.18,19
Several studies have shown successful ‘off-label’ use of TAVR for the treatment of AI in non-LVAD patients.16–18 Newer generation valves are now developed with special focus on treating severe AI.20,21 More recently, several small-sized single-centre studies have also reported successful ‘off-label’ use of TAVR among LVAD patients with post-implant AI.5,16,18,22,23 Phan et al., in a review of 29 published cases of percutaneous therapies for post-LVAD AI, reported that eight patients underwent TAVR, two of whom suffered device migration and one of whom experienced severe paravalvular regurgitation; cumulative survival at 20 months post-TAVR in that cohort was 35%.24 Yehya et al., from a single-centre series of nine patients undergoing TAVR for post-LVAD AI, reported 100% in-hospital and 89% 6-month survival.5
In the national cohort of patients in our study (n=105) who underwent TAVR, we observed a significantly lower risk of perioperative complications, including mortality, when compared with SAVR (Table 2). Of note, the prevalence of in-hospital mortality was <5% in the TAVR group. Similar to prior reports, we observed a statistically non-significant, but higher, risk of paravalvular regurgitation in our cohort. Although the precise reasons for superior short-term outcomes with TAVR compared with SAVR cannot be elucidated from this study, our findings suggest that addressing post-LVAD AI percutaneously and before it leads to other complications may be warranted.
Study Limitations
Our study had several limitations, including those associated with national registry analyses. The NIS only reports outcomes during index hospitalisation; hence, conclusions regarding long-term outcomes and prognosis cannot be determined from these data. It also lacks the clinical course of patients leading up to the index hospitalisation, including chronicity of their aortic regurgitation, right ventricular function, severity of heart failure symptoms, prior complications related to LVAD, duration of LVAD support and any prior medical interventions for aortic regurgitation. Furthermore, this database lacks granular patient data, including baseline haemodynamics, echocardiographic, computerised tomography scan, laboratory data and initial indication for LVAD (i.e. bridge to transplantation versus destination therapy), device type (HeartMate 2, HeartMate 3 or HeartWareTM), procedural details for TAVR or SAVR, as well as psychosocial determinants of outcomes. Other potential limitations of our study include a possibility of selection bias based on availability of centre-specific technical expertise.
Conclusion
In this nationally representative cohort of LVAD patients with post-implant AI, we observed that TAVR was associated with significantly lower risk of adverse short-term outcomes compared with SAVR. Future studies focusing on appropriate patient selection, timing of intervention, intraprocedure haemodynamic optimisation and long-term outcomes are warranted.
Clinical Perspective
- Post-LVAD aortic insufficiency is a known sequela of prolonged continuous flow left ventricular support with significant impact on patient outcomes.
- Limited data are available regarding outcomes with surgical versus transcatheter approaches for the management of post-LVAD aortic insufficiency.
- In this nationally representative cohort of LVAD patients with post-implant AI, we observed that TAVR was associated with a lower risk of adverse short-term outcomes compared with SAVR, and may be a viable option for treatment of post-LVAD AI.