Aortic stenosis (AS) is one of the most prevalent valvular diseases and the most common indication for valve intervention.1 AS leads to an increase in left ventricular (LV) compliance, LV and left atrial (LA) filling pressures, and a reduction of LV contractility and cardiac output.2 In parallel, subendocardial ischaemic damage and fibrosis contribute to pump failure.3 In aortic regurgitation, chronic volume overload gradually results in LV dilation and remodelling, leading to LV decompensation with dysfunction at late stages and heart failure.
The onset of symptoms is an indicator of a grave outcome and only timely aortic valve intervention (surgical or transcatheter) improves patient prognosis and quality of life.4,5 The gold standard valvular disease management involves a multidisciplinary heart team strategy where the cardiac imaging expert has a crucial role.4
Echocardiography is the key imaging modality for the assessment of valve morphology, severity and mechanism of the valvular lesion, haemodynamic consequences and suitability for transcatheter intervention.
Stress Echocardiography in Aortic Stenosis
Stress echocardiography has an established role in excluding ischaemia as well in as the dynamic evaluation of LV interaction with valvular structures and function during physiological (exercise) or pharmacological (inotropes and vasodilator) stress.5,6
Current guidelines recommend stress echocardiography when there is a discrepancy between the presenting symptoms and the findings of baseline echocardiography as well as in patients with asymptomatic severe AS who may not be aware of subtle changes to their effort tolerance.2,4 This assessment may play a central role in refining the timing of surgical or transcatheter intervention.
Severe symptomatic AS is associated with a high mortality of up to 50% at 1 year and patients with an abnormal stress echocardiogram have an eight times higher risk of major adverse cardiac events (including sudden cardiac death) and an increased risk of mortality in the longer term.4
Stress echocardiography can be helpful in several challenging scenarios as described below.
Asymptomatic Severe Aortic Stenosis
Exercise stress echocardiography can unmask symptoms and demonstrate markers of poor prognosis, such as an abnormal blood pressure response, reduced LV contractile reserve or induced pulmonary hypertension (pulmonary arterial systolic pressure >60 mmHg).
In conjunction with serum biomarkers (e.g. elevated B-natriuretic peptide), this investigation may help to restratify a patient’s risk of major adverse events and identify the optimal timing for intervention.5–7
Aortic Stenosis with Low-flow, Low-gradient and LV Dysfunction
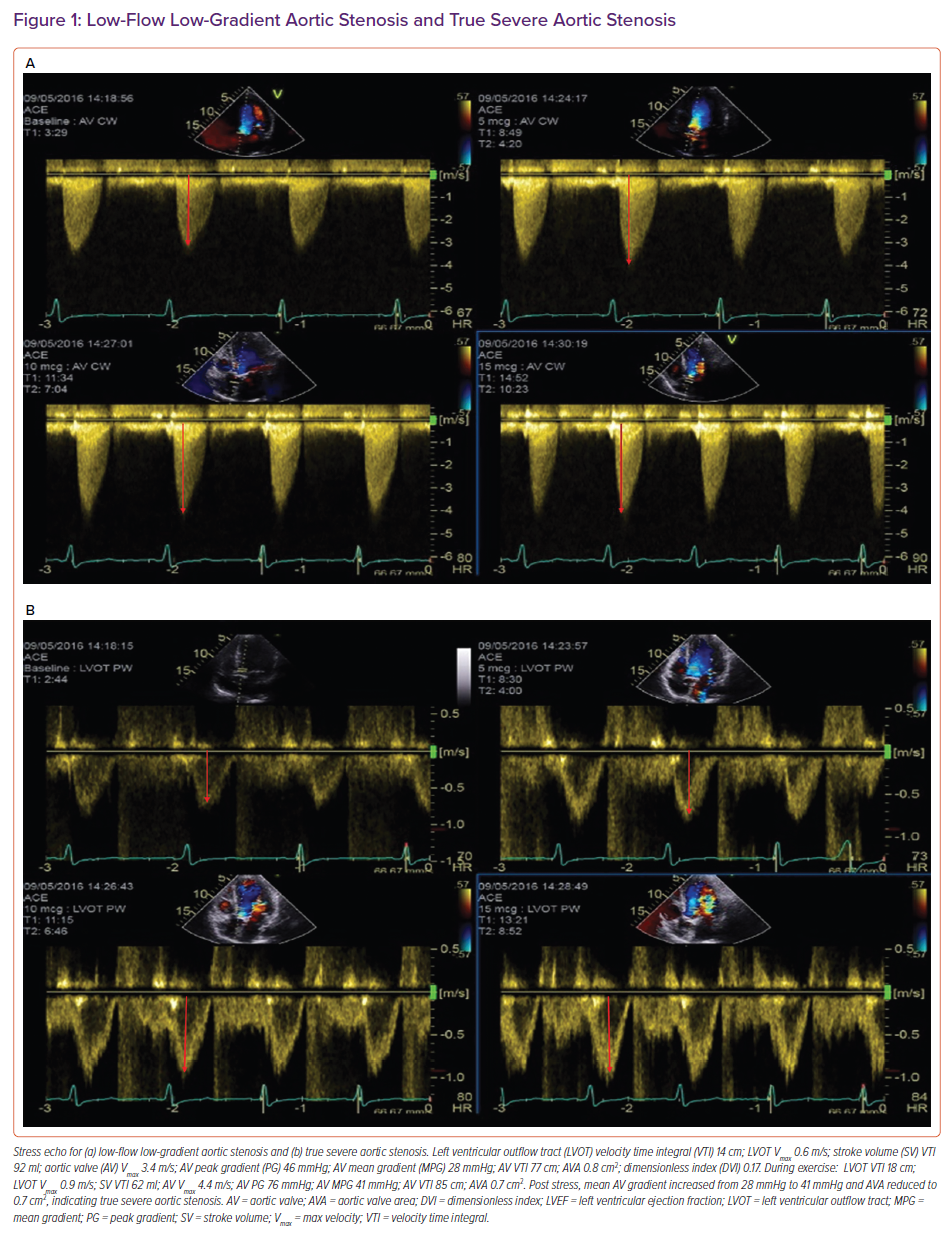
True aortic stenosis with low-flow, low-gradient (LFLG) and LV dysfunction (aortic valve area [AVA] <1.0 cm2; flow <35 ml/m2; gradient <40 mmHg; LV ejection fraction [LVEF] <50%) arises as a result of a fixed, narrow aortic valve area causing rising afterload, declining ejection fraction (EF) and reduced stroke volume. It can be reversed by valve intervention.
Pseudo-severe AS results from submaximal opening of the valve because of a reduction in the opening force because the left ventricle is impaired.7,8 Low-dose dobutamine stress echocardiography can differentiate between true severe LFLG AS and pseudo-severe AS by identifying increasing flow reserve (stroke volume >20%) with proportional changes in transvalvular flow.9
Patients with true severe LFLG AS are able increase their mean gradient >40 mmHg while maintaining an AVA at <1.0 cm2. In contrast, an increase in transvalvular mean gradient (>40 mmHg) increases the AVA to >1.0 cm2 in patients with pseudo-severe AS because better opening forces are generated by stronger ventricular contractility.
An illustrative example of a true severe and pseudo-severe LFLG AS is demonstrated in Figure 1.
Aortic Stenosis with Low-flow, Low-gradient and Preserved LV Function
Aortic stenosis with LFLG and preserved LV function (AVA<1.0 cm2; flow <35 ml/m2; gradient <40 mmHg; LVEF >50%) is also known as paradoxical LFLG.
This pathophysiological entity is characterised by a paradoxically low gradient (<40 mmHg) despite preserved LV function.2,5,6 It has similarities with heart failure (HF), with preserved EF, impaired diastolic function due to exaggerated concentric myocardial remodelling, reduced LV cavity size and compliance, increased global LV afterload and reduced myocardial contractility.
There is insufficient evidence regarding the safety of stress echocardiography in these patients (Figure 1) and recent ESC guidelines have advocated integrated assessment using cardiac CT calcium scoring of the aortic valve.4
Echocardiography in Transcatheter Aortic Valve Interventions
Transcatheter aortic valve intervention (TAVI) has become a standard, low-risk procedure for managing older patients with severe AS and high surgical risk. Recently, the indications for TAVI have expanded to cover all risk groups based on encouraging results concerning the durability and clinical outcome of new-generation TAVI valves.10–12
Although multidetector cardiac CT is the preferred imaging modality for pre-procedural TAVI workup, there is still a role for transoesophageal (TEE) imaging as a highly accurate alternative.13 This includes situations when iodinated contrast is not desirable (because of severe renal insufficiency or contrast allergy) or when CT images are suboptimal.
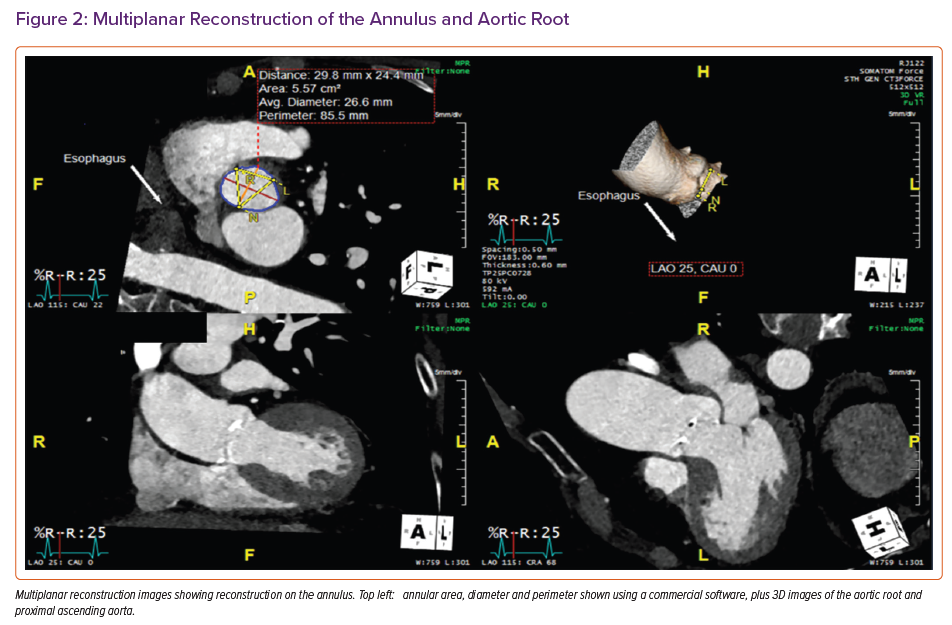
The virtual aortic annulus can be precisely traced from the most basal cusp insertion point using multiplanar reformation (MPR) based on 3D TEE acquisition (Supplementary Figure 1). Aortic annular dimensions and the height of the coronary ostia can be measured to predict the risk of obstruction. Four views are employed: sagittal; coronal; transverse; and a full-volume render.
The mid-systolic frame should be selected and then alignment of the crosshairs in sagittal and coronal planes along the long axis is important. Subsequently, the transverse plane should be aligned at the level of the annulus (the hinge point of the three cusps). Rotating the plane is important to confirm that the transverse view is bisecting the hinge point at the level of the non-coronary and left coronary cusps. The annulus circumference and area can then be traced in transverse view at inner edge. To measure the annulus to the left main coronary height (<11 mm is considered to show a high risk of coronary obstruction), the sagittal plane is advanced cranially until the origin of the left main stem (LMS) is identified, usually at the 10 o’clock position of the transverse image. The transverse image is then rotated anticlockwise until it is aligned with the LMS ostium. The distance from the base of the left coronary cusp to the ostium can then be measured.
Adhering to periprocedural TEE guidance is essential for anatomically challenging TAVI cases.14 This allows accurate positioning and deployment of the TAVI valve, assessment of paravalvular regurgitation and prompt detection of complications such as aortic tear or pericardial effusion (Supplementary Figure 2).14
TAVI for isolated aortic regurgitation is not as well established but may be offered to candidates with prohibitive surgical risk after detailed imaging evaluation.15–18 Potential problems include dilation of the aortic root and annulus, and insufficient leaflet and annular calcification to anchor the valve posing risks of transcatheter valve embolisation, migration and significant paravalvular regurgitation.15,16
Newer-generation devices, such as the Helio transcatheter aortic dock (Edwards Lifesciences), have shown encouraging results with better procedural outcome and less residual paravalvular regurgitation.19 The native aortic valve cusps are generally caught between a dock pre-placed in the aortic root, followed by TAVI to provide better fixation and paravalvular sealing. Another new device, the JenaValve prosthesis (JenaValve Technology), uses clip fixation to the native aortic valve cusps to secure TAVI positioning.20
Transcatheter valve-in-valve implantation is an alternative to standard redo surgery for failing bioprostheses and has been used successfully in selected high-risk patients.4 The risk of coronary artery obstruction and patient-prosthesis mismatch are important considerations, especially in smaller surgical bioprostheses.4
Pre-procedural CT is usually carried out for detailed assessment of the aortic annulus and bioprosthetic valve dimensions. In additional, periprocedural TEE is essential to guide optimal valve positioning, especially in the absence of significant calcification or when stentless valves make fluoroscopic visualisation challenging.13
CT in Transcatheter Aortic Valve Interventions
Pre-procedural imaging with CT focuses on the assessment of aortic valve morphology and the aorta, iliac and femoral arteries to provide information concerning vascular access.21 In addition, assessing aortic valve calcifications using non-contrast CT helps to confirm true severe aortic stenosis in challenging low-gradient scenarios. The threshold for highly likely severe stenosis is >3,000 Agatston units for men and >1,600 for women.21
The CT TAVI protocol at our centre consists of a single systolic phase acquisition of the aortic valve if the patient is in sinus rhythm (or multiphase retrospective ECG-gated acquisition in AF), immediately followed by a high-speed dual source acquisition of the aorta from the clavicles to the femoral heads to assess the whole aorta and iliofemoral vessels. Aortic valve measurements are obtained from systolic phase reconstruction, usually at 20% of the cardiac cycle, in line with recent guidance showing that aortic dimensions are slightly larger in systole. A different transcatheter valve sizing algorithm has been developed and described using systolic data.21,22
The fundamental measurements required for TAVI include annular area, perimeter and axial diameters to allow accurate TAVI sizing (Figures 2 and 3). Defining the extent of calcification within the annulus (and possible extension into the left ventricular outflow tract [LVOT]) is also important because this is associated with a higher risk of annular rupture or paravalvular regurgitation. For the same reason, the presence and extent of mitral annular calcification should also be described.22
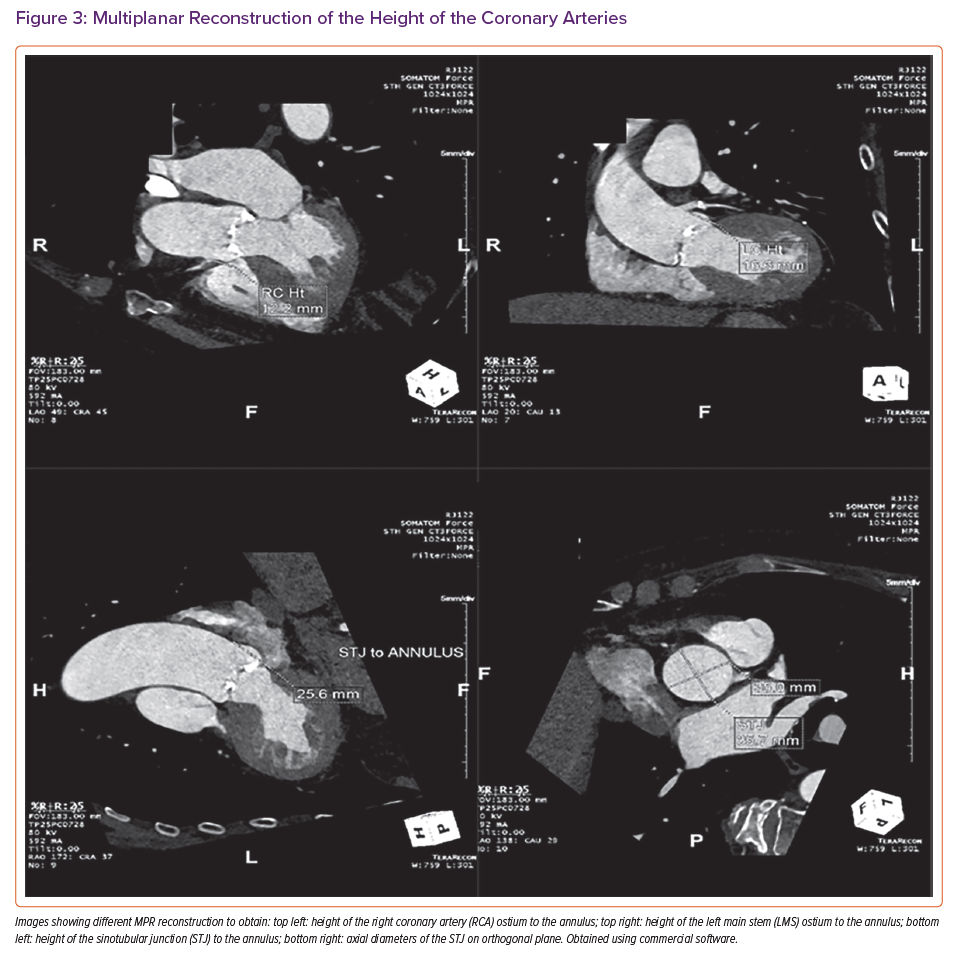
Measurements of the aortic root are also important to guide TAVI procedures. These consist of the diameters of the sinus of Valsalva and the sino-tubular junction, and the height of the coronary ostia above the annulus to guide transcatheter heart valve sizing and positioning and identify any risk of coronary occlusion.23,24 CT can also be used to identify the optimal fluoroscopic projections for valve deployment.
The whole aorta is then assessed (with particular attention to the ascending aorta) for any evidence of calcification, thrombus, aneurysm or dissection. Ascending aorta diameters are usually measured at the level of the main pulmonary artery (or at its greatest width).
Finally, the presence or absence of coronary artery disease can be assessed opportunistically, although this can prove technically challenging in the presence of severe coronary calcification or arrhythmias (Figure 3). Beta-blockers and nitrates, which are used in standard CT coronary angiography, are usually contraindicated in severe AS.
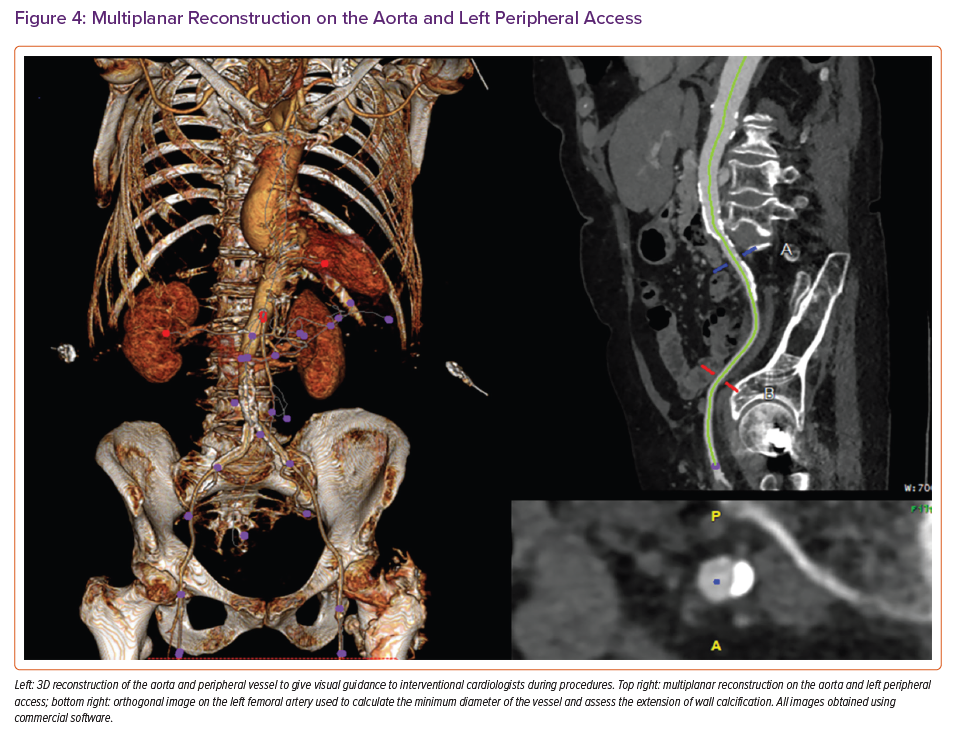
Evaluation of the peripheral vessels is essential to establish the site and height for best access for TAVI (Figure 4). The minimum luminal diameter and extent of calcification are measured and commented for both common iliac, external iliac and common femoral arteries and the femoral bifurcation. The tortuosity index, which is the ratio of the length along the centreline of the vessel to the linear distance between the two endpoints, is calculated by automated software for both sides.
The recommendation for optimal access side favours that with larger diameters, fewer calcifications and less iliac vessel tortuosity. Comments concerning the subclavian or carotid arteries or inferior vena cava should also be made when these approaches are being considered. Finally, extra-cardiac findings are reported by a radiologist according to local protocol.24
It is important to note that CT TAVI is a complex examination from initial patient selection and image acquisition to final reporting. The entire process requires close collaboration with an experienced multidisciplinary team to obtain optimal information for procedural planning.
Cardiac Magnetic Resonance in Aortic Valve Disease
Cardiac magnetic resonance (CMR) has not been routinely employed in AS but is emerging as an important component of multimodality imaging assessment because of the high degree of amyloid build-up in elderly TAVI patients.25–27
CMR is the gold standard for evaluation of LV volumes, EF and mass.26 It is also important in the characterisation of concentric or asymmetric hypertrophy. In one study, asymmetrical wall thickening was identified in 27% of patients with AS, leading to new classification consisting of six patterns of LV remodelling being proposed: normal geometry; concentric remodelling; asymmetrical remodelling; concentric hypertrophy; asymmetrical hypertrophy; and LV decompensation (eccentric hypertrophy).27
In patients with discrepant transthoracic echocardiography (TTE) data, CMR is useful in measuring aortic valve area by planimetry. CMR planimetry of the aortic valve area is reproducible, observer independent and correlates well with both transoesophageal echocardiography measurements and Gorlin method.
In patients with bicuspid valve AS, ascending aortic dilatation is common. CMR can not only allow better visualisation of aortic valve morphology but also is a great modality for aortic measurements, particularly with difficult TTE images.
Finally, accurate measurement of aortic annulus can be difficult in patients with advanced kidney issues, and non-contrast CMR is a safer alternative for aortic annulus measurements.
CMR can also be considered as a promising alternative for planning valve in-valve procedures in patients with bioprosthesis and advanced kidney dysfunction
CMR is the only non-invasive modality to assess myocardial fibrosis using either late gadolinium enhancement (LGE) to quantify focal interstitial expansion or T1 myocardial mapping to characterise diffuse interstitial expansion.
The pattern of fibrosis in aortic stenosis is distinct. Mid-wall scarring with focal fibrosis is typical and strongly associated with adverse outcomes, including death, myocardial injury, and systolic and diastolic dysfunction.28,29 Contrast-enhanced T1 mapping does not rely on contrasting signal intensity and allows quantification of diffuse fibrosis, which is also associated with adverse events.30
Typical CMR findings of cardiac amyloidosis in AS patients include diffuse subendocardial or transmural LGE and elevated native T1 and extracellular volume on T1 mapping sequences.
Finally, CMR is useful in assessing the aetiology of LV dysfunction in patients with LFLG AS.
Mitral Valve Disease: Regurgitation
Mitral regurgitation (MR) is a common valvular lesion and secondary MR (SMR) is present in up to 25% of patients with heart failure (HF) and is associated with a poor prognosis.31
Both well-established and novel transcatheter treatment options are available for anatomically suitable patients with severe MR who are at high surgical risk and remain symptomatic despite optimal medical therapy.
Echocardiography in Transcatheter Mitral Valve Interventions
Two- and 3-dioptre echocardiography is the standard modality to elucidate the cause and mechanism of MR, provide specific measurements, assess haemodynamic consequences and determine any anatomical challenges, which are crucial to know when assessing the feasibility of surgery or transcatheter intervention.
Structured TEE with 3D, multiplane 2D and colour modes provides detailed anatomical assessment of prolapsed/flail/cleft mitral valve segments and the sub-valvular apparatus, and enables quantification of regurgitant jets including direct planimetry of the vena contracta area using MPR for more accurate measurement of the non-circular regurgitant orifice in SMR.13,32 3D transthoracic and TEE elucidates the mechanism of mitral valve disease and highlights the anatomy of the mitral valve.
The following are important considerations in TTE and TEE for mitral valve disease:
- Mitral valve anatomy: leaflets, mitral annulus, aortomitral continuity, and posteromedial and anterolateral commissures;
- Chordae tendinae;
- Papillary muscles;
- LV size and function;
- Left atrial size;
- Right heart assessment for evidence of pulmonary hypertension;
- Global longitudinal strain.
For mitral stenosis, assessment with the Wilkins score determines the suitability of the mitral valve for percutaneous mitral balloon valvuloplasty. Poor valve morphology can lead to severe mitral regurgitation after intervention. The score is based on leaflet mobility, valve thickness, subvalvular thickening and valvular calcification.32
Stress Echocardiography in Mitral Valve Disease
Stress echocardiography in mitral valve disease can be performed either as exercise or dobutamine stress echocardiography depending on clinical status and the severity and type of valve disease.33,34
Exercise stress echocardiography provides an objective assessment of symptoms at different exercise levels, which is of prognostic importance in patients and enables better treatment planning.
Dobutamine stress echocardiography clarifies valve condition severity and helps in assessing surgical risk in patients with systolic dysfunction. It can be used to assess valve haemodynamics in asymptomatic patients with significant mitral stenosis who are unable to perform an exercise test, or to assess left ventricular contractile reserve and viability in patients with ischaemic secondary mitral regurgitation.
Mitral transcatheter interventions
Mitral Edge-to-edge Repair
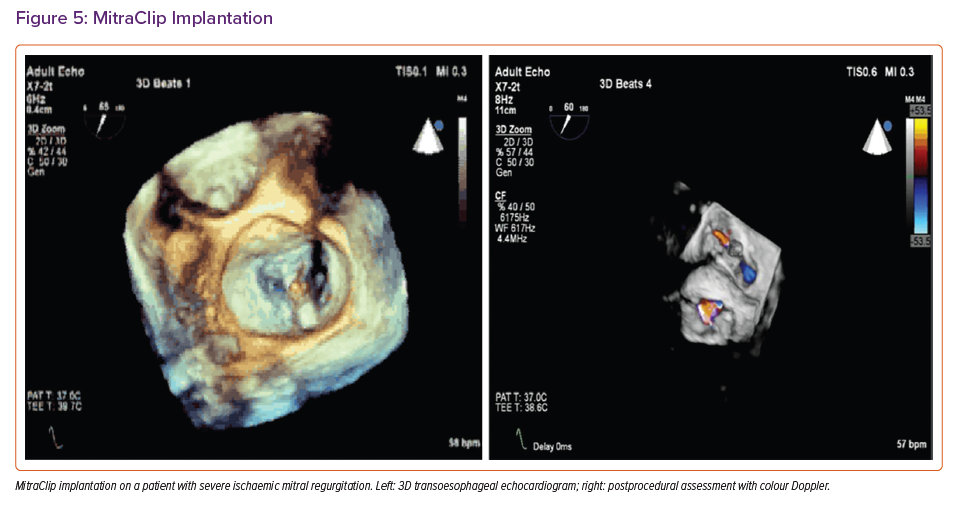
The MitraClip device (Abbott) is most widely used for transcatheter mitral edge-to-edge repair (TEER) and is known to improve symptoms and reduce HF hospitalisations in anatomically suitable candidates with severe primary or SMR and symptomatic HF if surgery is contraindicated or high risk.35
Careful 2D and 3D echocardiographic assessment can identify subjects with symptomatic HF and disproportionate SMR (Supplementary Table 1) who can be expected to benefit most from TEER (Figure 5).4,6,35 Careful pre-procedural assessment with TEE is also essential to assess suitability based on favourable anatomical characteristics (Supplementary Table 2). Adherence to TEE guidance is necessary for each step of a successful procedure and prompt detection of complications (Supplementary Table 3).
With increasing global experience, new designs (e.g. Pascal, Edwards Lifesciences) and an increasing range of device sizes, more complex TEER is increasingly performed in patients with less favourable anatomy with high success rates.
Transcatheter mitral valve replacement
Transcatheter mitral valve replacement (TMVR) is increasingly used to treat significant MR affecting native valves, dysfunctional annuloplasty repairs or degenerating bioprostheses in selected patients at prohibitive surgical risk.
Both purpose-built and repurposed transcatheter aortic valves have been used with promising early outcomes.36,37 Important issues associated with TMVR include the risk of valve protrusion to cause LVOT obstruction, adequate valve anchoring, annular retention and risk of paravalvular leak (PVL). Given the complexity of the procedure and the variability of anchoring and delivery, a multimodality approach allowing detailed assessment of the mitral valve and LV anatomy is required for patient selection.13
Peri-procedural TEE guides the transseptal or transapical puncture, safe device advancement, correct positioning and valve deployment, followed by assessment for PVL and residual atrial septal defects (with consideration of closure when appropriate) and the detection of potential complications, including valve migration or LVOT obstruction (Supplementary Figure 3).37–39
Cardioband transcatheter annuloplasty ring
The Cardioband transcatheter annuloplasty ring (Edwards Lifesciences) has been used to reduce annular dilatation and SMR with promising results.40,41 2D/3D TEE and CT are essential for pre-procedural planning to determine whether there is sufficient annular tissue for device anchoring and a safe distance from the left circumflex coronary artery.40
2D multiplane and live 3D TEE images help to identify the optimal transseptal puncture site and guide accurate positioning of the delivery system at the mitral annulus followed by anchor deployment.41
3D TEE with MPR is then used to measure the newly formed mitral annular dimensions and assess valve and mitral orifice area after annular contraction to ensure optimal MR reduction without increasing trans-mitral gradients.41
CT in Transcatheter Mitral Valve Interventions
Cardiac CT is paramount in pre-procedural assessment with advantages including superior spatial resolution and the ability to perform multiplanar reconstructions and assessment of surrounding structures.42,43
The CT TMVR protocol at our centre consists of a multiphase retrospective ECG-gated acquisition of the whole heart, allowing measurement of mitral annular dimensions (including trigone-to-trigone distance), septal-lateral and inter-commissural distances, and annular area. These will guide TMVR device sizing and avoid embolisation or annular rupture (due to undersizing or oversizing, respectively).
Reconstruction of the 3D, saddle-shaped mitral annulus can be performed on dedicated software to further facilitate accurate measurement and device sizing. Different devices require different measurements of mitral annular dimensions; for example, SAPIEN 3 (Edwards) valves use mitral annular area whereas Tendyne (Abbott) valves use inter-commissural and septo-lateral distances.44,45
CT also allows optimal sizing of valves for mitral valve-in-valve or valve-in-ring procedures and retrospective analysis can be helpful in evaluating prosthetic valve positioning and leaflet motion. It can also differentiate between thrombus and pannus on an existing prosthesis to guide decisions concerning anti-thrombotic therapy, and provide detailed information concerning the size and location of any PVL prior to device closure.
LVOT obstruction is an important complication of TMVR and 3D or 4D cardiac CT reconstructions using dedicated software allow prediction of the neo-LVOT area and selection of the most appropriate device for an individual patient.44,45 Measurement of the smallest neo-LVOT cross-sectional area is taken in end-systole and an area >200 mm2 considered favourable (although no validated cut-offs yet exist). The risk of neo-LVOT obstruction was deemed higher with an area <190 mm2 in one study and <170 mm2 or <60% in others.46 Other contributory aspects include basal septal hypertrophy, small LV cavity and acute aortomitral angle.
Cardiac CT plays a role in assessing the anatomy and proximity of the left circumflex coronary artery and coronary sinus to the mitral annulus to establish any risk of compression of these structures with device oversizing. CT can also help in planning access (transapical, transeptal or, rarely, transatrial) and provide the optimal fluoroscopic angle for deployment.46. Transeptal, transvenous access is typically achieved through the femoral veins, which can be identified using dedicated venous phase imaging.
Cardiac Magnetic Resonance in Mitral Valve Disease
Current guidelines for the assessment of MR emphasise the importance of evaluating the severity of regurgitation alongside the effect on LV and LA remodelling.47 CMR is a highly accurate and precise modality for estimating heart chamber volumes and function, and the gold standard for assessment of LV volumes and systolic function along with MR severity.
Cine imaging using ECG-triggered, steady-state, free-precession or fast-spoiled gradient echo sequences is used to scan the heart in multiple planes irrespective of body habitus, offering an unparalleled tool to visualise the regurgitant lesion and supporting TTE in defining its mechanism(s).48
Cine imaging also allows measurement of LV volumes, LV stroke volume and EF by segmenting a base-to-apex stack of cine images in end-diastole and end-systole, and applying Simpson’s method, which is independent of any assumption related to chamber geometry.49
A similar approach can be used to measure left atrial volume and function. Cine imaging is complemented by phase-contrast, velocity-encoded imaging, which serves to enhance visualisation of the regurgitant lesion when acquired along the direction of the jet (in plane) or to quantify flow through the ascending aorta when images are taken perpendicular to the vessel (through plane).
Regurgitant volume (RVmitral) and fraction (RFmitral) methodology is independent of other regurgitant lesions, including aortic or tricuspid regurgitation, or any intra-cardiac shunt. The CMR measurement of mitral regurgitant volume is reproducible and can measure the severity of MR regardless of jet morphology even in cases of eccentric or multiple jets.
Multiple studies have now not only confirmed the accuracy and reproducibility of CMR in mitral regurgitation quantification but also shown prognostic data associated with MR severity.
Preliminary evidence suggests poor agreement in the assessment of MR severity using CMR and TTE.48 However, the presence of a flail leaflet and Coandă effect on TTE in patients with primary MR has been associated with greater RVmitral and RFmitral.49
Besides this indirect method, techniques directly gauging the regurgitant volume have also been implemented, including signal void, the size of the regurgitant jet and the regurgitant orifice area.50
Tricuspid Valve Disease: Regurgitation
Significant TR and its outcomes have increasingly been studied in recent years, with the recognition that moderate or great TR is associated with excess mortality.51
Secondary TR is the most frequent aetiology but few patients undergo surgery as recurrence rates are high an clinical outcomes poor.52 However, the advent of transcatheter therapies has allowed carefully selected patients to be treated without open heart surgery.
Imaging plays a key role in patient selection and procedural planning before transcatheter tricuspid valve repair and replacement. The tricuspid valve has a saddle-shaped, elliptical annulus and a variable number of leaflets. A recent TEE study showed that only 57% of tricuspid valves are composed of three leaflets (anterior, septal and posterior).53 Important adjacent anatomical structures include the right coronary artery, non-coronary sinus of valsalva, atrioventricular node and His bundle, and inferior and superior vena cava.54
TTE and/or TEE are the gold standard methods for evaluation of the mechanism and severity of TR (Supplementary Figure 4). Semi-quantitative assessment using colour and spectral Doppler does not reliably reflect the regurgitant volume and quantitative methods are essential.
Although severe TR is defined as an EROA (effective regurgitant orifice area) ≥0.40 cm2 and regurgitant volume ≥45 ml in the American and European guidelines, a wide systolic gap between leaflets and hepatic flow reversal (assessed with pulsed-wave Doppler and a triangular continuous-wave Doppler signal) are specific signs of severe TR (and supported by right atrial and ventricular dilatation).4,6,55
TEE assessment of valve morphology and severity of TR are essential before the procedure (Supplementary Figure 5). Since the tricuspid valve is located anteriorly, deep oesophageal and transgastric views should be included during work-up to improve leaflet visualisation. Simultaneous demonstration of three leaflets in the 3D en face view allows localisation of the regurgitant jet, which is especially important when planning tricuspid edge-to-edge repair.
Optimal evaluation of lead impingement is also achieved in this view.55–57 Right ventricular (RV) dilatation is common in patients with chronic significant TR and right ventricular dysfunction indicated by a tricuspid annular plane systolic excursion (TAPSE) <17 mm, fractional area change <35%, tricuspid annular systolic velocity wave (S’) <9.5 cm/s, Tei index >0.43 and RV longitudinal strain ≥20.57,58
Advanced Echocardiography and CT in Tricuspid Structural Interventions
Several options for transcatheter tricuspid intervention are under active investigation: edge-to-edge repair; annuloplasty; caval implants; spacer; and valve replacement.57
Thorough imaging work-up is essential for procedural success and right heart catheterisation is helpful to exclude pre-capillary pulmonary hypertension. TTE and TEE provide the following information:
- RV volumes and ejection fraction;
- RV dimensions (from all possible views);
- TR quantification;
- Tricuspid leaflets and subvalvular apparatus;
- Tricuspid annular assessment (area and perimeter for appropriate valve sizing);
- Coaptation gap;
- Right coronary anatomy;
- Course of the superior and inferior vena cava;
- Lead impingement in patients with a permanent pacemaker or implantable defibrillator.
Edge-to-edge Repair
Imaging requirements before and during tricuspid edge-to-edge repair are summarised in Supplementary Tables 4 and 5.59,60 TEE assessment of the final result is essential to ensure correct device positioning and the degree (and mechanism) of any residual regurgitation.
TTE is the best imaging modality to assess RV volumes, EF and the degree of residual regurgitation over long-term follow-up, although CT or CMR may be useful to detect evidence of any shunts or device migration if there is a suspicion of complications.
Tricuspid Annuloplasty
This technique is most suitable when severe TR is related to tricuspid annular dilatation with or without a coaptation gap (if present, this should be <10 mm).
The Cardioband device consists of a flexible ring with multiple anchors attached to the annulus. In contract, the Trialign (Mitralign) device results in a bicuspid tricuspid valve and a reduction in annular dimensions.60,61
CT is important in assessment for access and documenting the right coronary artery anatomy since iatrogenic dissection is a rare but potentially serious complication.
Recent reports have recommended performing annuloplasty in two stages to secure better long-term results.62 Overall, tricuspid annuloplasty is preferable when there is RA dilatation, smaller RV dimensions and favourable right coronary artery anatomy coursing along the atrioventricular groove.
Caval Implants
The superior and inferior vena cava are of paramount important and easily assessed using CT. The distance between the cavoatrial junction and the first hepatic vein is critical, as well as right ventricular systolic function and right atrial size (particularly for the Tricento device).63
Severe RV dysfunction and significant caval dilatation prohibit this approach, although it remains an attractive option for patients with pacemaker-lead-induced severe TR.
Spacer Implants
Spacer devices, such as the Forma (Edwards Lifesciences), aim to minimise the leaflet coaptation gap caused by tricuspid annular dilatation and leaflet tethering using a foam-filled polymer balloon (spacer) deployed at the level of the tricuspid valve and an anchoring system placed in the right ventricular septal free wall groove.63
Favourable anatomy is indicated by a small coaptation gap (<7 mm) and a large tricuspid annulus; the procedure cannot be carried out in the presence of venous occlusion or pacemaker leads. CT assessment is crucial for the assessment of right ventricular anatomy and the course of the axillary and subclavian veins.
Tricuspid Valve Replacement
Suitable patients include those with a large coaptation gap (>7–10 mm) and severe tethering (>10 mm), and those who have undergone previous surgical treatment with a bioprosthetic valve or ring.60
Again, CT is important in the assessment of the right ventricle and surrounding anatomical structures, as well as in identifying the mechanism of valve failure and the dimensions of any previously implanted valve or ring.64–66
The high spatial resolution of CT provides essential information concerning RV volumes and function, and newer imaging sequences have significantly reduced breath holding, radiation dose and contrast-induced nephropathy.
Additional information concerning surrounding anatomical structures, vascular access and tricuspid annular morphology, commissural location, tethering height and angle, and device landing zone is key to determining successful procedural outcome.
For example, CT can provide 3D sequences of the saddle-shaped tricuspid annulus and highly reproducible measurement of the tricuspid valve orifice area.65,66
Cardiac Magnetic Resonance
CMR allows the accurate assessment of RV volumes, EF and mass in patients with significant TR, and may also provide accurate assessment of tricuspid morphology and regurgitation, using direct (RV and LV stroke volume) and indirect (phase-contrast imaging) methods.67
Identifying the extent of RV fibrosis is useful in assessing the risk of adverse outcomes when there is suspicion of underlying cardiomyopathy or pulmonary hypertension.67
Pulmonary Valve
Structural interventions are usually needed in patients with congenital heart disease such as in those with residual stenosis, regurgitation or mixed disease of the pulmonary valve following repair of congenital right ventricular outflow tract (RVOT) abnormality.
Transcatheter pulmonary valve replacement is an alternative to surgery in patients with dysfunctional right ventricle-pulmonary artery conduits or bioprosthetic valves.68,69 It is also useful in patients who cannot undergo open-heart surgery, such as pregnant women.70
TTE and TEE remain the first-line imaging modalities for the assessment of transcatheter gradient or evidence of patient prosthesis mismatch. However, the imaging of the pulmonary valve can often be technically challenging. In these patients, CMR remains gold standard for the assessment of RV volumes and EF, as well as RVOT assessment.
CT is also an important part of percutaneous structural interventions to assess anatomy and surrounding structures, and also an accurate way to assess leaflet motion.68 3D printing is an evolving hybrid imaging modality which is key in challenging structural interventions.69
Conclusion
Multimodality imaging and evaluation of patients with valvular heart disease earlier than later are crucial for the success of structural interventions.
Establishment of heart centres with multimodality imaging available that take a heart team approach which includes an imaging cardiologist is essential as the new indication for percutaneous therapies continue to expand and evolve.