Historically overlooked and under-reported in women, cardiovascular disease (CVD) is a major cause of death in women.1 Some of the most significant sex-related differences in CVD have been found in heart failure (HF). Although the overall lifetime risk of HF seems comparable between men and women, women have better survival rates but worse quality of life.2,3 Women also have a higher prevalence of HF with preserved ejection fraction (HFpEF) and this trend becomes greater with increasing age.4 Nevertheless, women are often underrepresented in clinical trials and therefore current evidence and recommendations do not necessarily reflect best practice for women.5 Several imaging societies have provided documents and recommendations to assess CVD in women, responding to the need for more sex-specific guidelines.6,7 The aim of this paper is to review sex-related differences and analogies in HF, highlighting the role of each imaging modality in the diagnosis and follow-up of women with HF.
Multimodality Imaging in Women: Sex-specific Features and Challenges, Particularly During Pregnancy
Some sex differences in the clinical features and outcomes of HF should be evaluated considering the existing characteristics observed even in healthy women hearts.6,7 In fact, despite no differences being observed in global left ventricular ejection fraction (LVEF) in a cohort of the Framingham Heart Study, women have smaller volumetric measures even after adjustment for body surface area and smaller indexed mass.8 Therefore, sex-specific normal reference ranges have been provided and should be systematically used in cardiac imaging assessment for women.9,10 Chung et al. have recently demonstrated that women have higher median (25th, 75th percentile) LVEF compared to men (75% [70, 79%] versus 70% [65, 75%], respectively, p<0.001), suggesting there is a need for sex-specific thresholds to define normal LVEF.11 Differences have been noted in how women’s hearts cope with haemodynamic overload. In fact, a significantly lower degree of left ventricular (LV) hypertrophy has been demonstrated at every stage of LV dysfunction in women compared to men.12. All this evidence suggests that women have unique anatomical and physiological features that should be considered during cardiological assessment (Table 1).
CVD affects 1–4% of all pregnancies and is the leading cause of pregnancy-related mortality in high-income countries.13 A review of cases of maternal mortality in the UK revealed that in 36% of cardiac-related deaths, improvements in care could potentially have changed the outcome.14 In particular, even when cardiac disease was suspected, pregnant women were less likely to have had access to standard investigations, such as CT scans, therapies, such as dual anti-platelets, or life-saving interventions such as electrical cardioversion or primary angioplasty, due to a significant overestimation of the risks to mother and foetus. Although in most cases, the symptoms of uncomplicated pregnancy and those of CVD can be differentiated through careful history taking, clinical examination and pregnancy-specific physiological parameters assessment, cardiovascular imaging plays a central role in evaluating women with symptoms and/or signs of heart disease in pregnancy. The goals of cardiovascular imaging in pregnancy are to rapidly achieve a definitive diagnosis, determine disease severity, risk stratify, assess treatment response and assist in the planning of the setting, mode and timing of delivery. The most appropriate imaging modality is the one that meets these goals, with a positive risk-benefit profile for the mother and foetus.
Transthoracic echocardiography (TTE) should be the first-line imaging modality, given its benign safety profile for mother and foetus – and its widespread availability. It is particularly useful in the assessment of pregnant women with disproportionate or unexplained dyspnoea or tachycardia or a new (pathological) murmur. Normal physiological changes of pregnancy include mild chamber dilatation and increases in transvalvular velocities and gradients. The use of echocardiographic contrast agents (ECA) can overcome some of the technical limitations of TTE, enhancing its diagnostic accuracy. These agents have outstanding scattering properties that improve the ability to delineate the endocardial border. They are considered to be very safe with a low reported risk of serious allergic reactions (approximately 1:10,000 doses).15 However, there are no studies investigating the feasibility and safety of the use of ECA in pregnant women and therefore their use in this subset of patients should be avoided, unless strongly necessary for diagnostic purposes.15 Transoesophageal echocardiography is relatively safe although the risk of vomiting and subsequent aspiration is higher in pregnant women.
Cardiovascular magnetic resonance (CMR) is safe during pregnancy. A large retrospective study demonstrated similar rates of stillbirth, neonatal death, congenital abnormalities, cancer or hearing loss in 1,737 pregnancies where CMR had been used during the first trimester compared to controls.16 CMR is indicated if echocardiography does not provide all the required information. It plays a particular key role when echocardiographic windows are poor, when highly accurate serial assessments of the heart and the aorta are required, perhaps to measure aortic dimensions in aortopathies and when multiparametric cardiac assessment is required, such as with differentiating myocarditis from MI. Long CMR protocols can be uncomfortable for patients, particularly in late pregnancy. Pregnant women should be imaged in a left lateral decubitus position to relieve compression of the inferior vena cava. There are no contraindications to MRI that are specific to pregnant women. However, the use of gadolinium contrast in pregnancy has been associated with a higher risk of stillbirth, neonatal death, infiltrative skin conditions, rheumatological and inflammatory disorders and should be limited to cases where its use can significantly improve diagnostic performance and is expected to improve foetal or maternal outcomes. Excretion of gadolinium-based agents into breastmilk is very limited (<0.04% of an IV dose within the first 24 hours, with 1–2% absorption) and therefore breastfeeding does not need to be interrupted after gadolinium administration.17
Non-invasive imaging is often used to differentiate ischaemic from non-ischaemic cardiomyopathy in HF patients, providing assessment of viability and ischaemia. This is particularly important in women, in whom a high discrepancy between symptoms and coronary artery disease (CAD) is often observed with obstructive CAD diagnosed only in a very limited percentage (~25%) of women undergoing invasive coronary angiography.18 CT angiography (CTA) is a useful non-invasive diagnostic tool for the evaluation of coronary anatomy. Meijboom et al. found a comparable sensitivity and negative predictive values for CT angiography in both women and men, but a lower sensitivity in detecting stenosis in distal segments and side branches in women.19 Nevertheless, CT angiography has the undoubted advantage of providing information about plaque characterisation with subsequent prognostic implications.20 Women with anginal symptoms also have a higher prevalence of ischaemia with non-obstructive coronary arteries (INOCA) compared to men (65% versus 32%, respectively).21 Despite the absence of obstructive CAD, INOCA is far from being a benign condition. A large study demonstrated that women with INOCA have a higher 1-year major adverse cardiovascular events (MACE) rate when compared with men or women with normal coronary arteries (threefold increase and 2.55-fold increase, respectively).22 Interestingly, this has also been associated with a 10-fold risk of hospitalisation for HF when compared to asymptomatic women.23 In this context, the already cited plaque characterisation by CT, the calcium score determination as well as myocardial perfusion quantification by MRI have demonstrated an additional role in defining prognosis.20,24,25
Single-photon emission computed tomography (SPECT) imaging can also be used to evaluate the presence of ischaemia. Moreover, 123-iodine metaiodobenzylguanidine SPECT has an established role in the evaluation of cardiac innervation in HF patients and it is globally reduced in these patients and is related to arrhythmic events.26 Ventilation-perfusion (V/Q) scanning and CT pulmonary angiography (CTPA) could be required to rule out pulmonary embolism in pregnant women presenting with acute HF.27 However, the use of radiation is often a major concern when imaging women, especially during pregnancy. The exposure to ionising radiation is associated with growth restriction, intellectual disability, malignancies and neurological effects, typically at doses of 100–200 mGy.28 Risks are highest in the first trimester during organogenesis and reduce as pregnancy progresses. However, with few exceptions, radiation exposure through radiography, fluoroscopy, CT or nuclear medicine imaging techniques is at a dose much lower than the exposure associated with foetal harm.28 If these techniques are required in addition to echocardiography or MRI, or are more readily available for the diagnosis in question, they should not be withheld during pregnancy. The general principle that ionising radiation doses must be kept as low as reasonably achievable (ALARA) applies and the radiation dose to the foetus should be kept as low as possible and <50 mGy. To put these doses into perspective, the foetal dose from a chest radiography is <0.01 mGy, a CTPA is 0.01–0.66 mGy and coronary angiography is 1.5 mG.29 Iodinated contrast agents should not be omitted in pregnant patients and breastfeeding can be continued without interruption.28
The key message for healthcare professionals is to investigate and treat women who are pregnant or who are breastfeeding in the same way as a non-pregnant person, unless there is a very clear reason not to. If considering deviations from evidence-based standard of care due to concerns regarding pregnancy or lactation, they should seek the support of their local pregnancy heart teams.
Heart Failure with Reduced Ejection Fraction
HF with reduced ejection fraction (HFrEF) is less common in women compared with HFpEF and this might explain the under-representation of women in HFrEF clinical trials.30 Women with HFrEF are more likely to have hypertension, valvular heart disease and diabetes and less likely to have AF and ischaemic heart disease compared to men with HFrEF.31,32 Although there are no significant sex differences in in-hospital mortality among patients with HFrEF, women with HFrEF are more symptomatic, with reduced 6-minute walk distances and a worse quality of life compared to men.33,34
There is evidence that women with HFrEF have higher ejection fractions, smaller left atria, higher longitudinal strain and higher circumferential strain.32,35,36 In contrast, another study showed no significant sex differences in LVEF.37 In women with HFrEF, tricuspid annular plane systolic excursion (TAPSE) and LV isovolumetric relaxation time were prognostic factors of mortality, whereas LV systolic function has better prognostic values for male stratifications.36
Considering that the LVEF cut-off value of 35% is crucial for decisions regarding revascularisation for coronary artery disease and device therapy for the management of HFrEF patients, as well as for therapeutic management and cardiac resynchronisation therapy (CRT), the precise quantification of LVEF is of extremely important in clinical practice.38,39 A study has shown that when LVEF is <35%, echocardiography significantly overestimates LVEF compared to CMR, which is the gold standard for LVEF quantification, although LVEF cut-off values in the guidelines are based on clinical trials with the assessment of LVEF by echocardiography.40 Although 3D echocardiography might overcome the limitations of 2D echocardiography in the assessment of LVEF related to geometric assumptions, the precise LVEF assessment by either method might still be limited by inaccurate tracing and load dependence.41
Unlike echocardiography, CMR has the ability to assess biventricular function and mass without making any geometric assumptions, therefore applying to even largely remodelled ventricles.42,43 The application of steady-state free-precession sequences (SSFP) enables the accurate assessment of regional wall motion abnormalities, particularly in patients with poor acoustic windows.42
Apart from the unique role CMR has in differentiating the ischaemic from non-ischemic cardiomyopathies with late gadolinium enhancement (LGE) imaging, it also predicts segmental contractile function post-revascularisation based on the transmural extent of MI and has become the routine diagnostic technique for the assessment of viability in clinical practice.44 Aneurysmal dilatation and thrombus formation as complications of MI can be detected by SSFP sequences and post-contrast inversion recovery sequences, respectively.42 It should be noted that the absence of coronary artery stenosis in an angiogram does not exclude the presence of ischaemic heart disease. A study showed that approximately 15% of patients with unobstructed coronary arteries had an ischaemic LGE pattern and they would have been misclassified as having non-ischaemic dilated cardiomyopathy (DCM) if CMR was not performed. This indicates the clinical significance of CMR in patients with MI with non-obstructed coronary artery disease.45
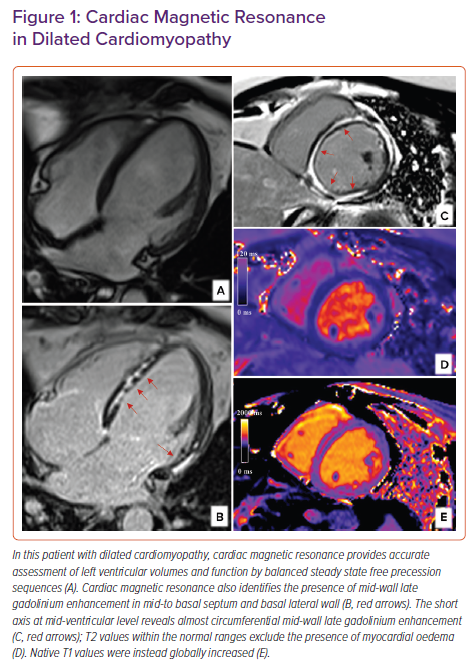
There is evidence that increased native T1, left atrium (LA) volumes and right ventricular (RV) dysfunction are independent predictors of survival and HF outcomes in patients with DCM.46–48 The presence of mid-wall LGE in 10–28% of DCM patients has been associated with an increased risk of hospitalisation for decompensated HF, sudden cardiac death, ventricular arrhythmias and all-cause mortality.49 Figure 1 demonstrates the unique role CMR has in tissue characterisation in a patient with DCM.
Heart Failure with Mildly Reduced and Preserved Ejection Fraction
Despite most of the evidence available regarding sex-related differences in HF being related to HFrEF, there is growing interest in sex-specific characteristics of patients with HF with mildly reduced EF (HFmrEF; defined as LVEF 41–49%) and preserved HF (HFpEF; LVEF >50%). Data coming from a recent meta-analysis including 4,458 women with an LVEF ≥45%, showed that women are more often older than men and more frequently affected by hypertension (86.6 versus 76.6%, respectively) and obesity (48.7 versus 41.2%, respectively). Interestingly, women complained about worse symptoms and worse quality of life despite lower levels of N-terminal pro B-type natriuretic peptide (NT-proBNP) and a higher LVEF.50 Women also had significantly lower rates of death but not of rehospitalisation; this different outcome could be in keeping with the lower rates of CAD noted in the women’s group.50 Echocardiography is usually the first recommended step of the diagnostic work-up of these patients and the assessment of the LV function is mandatory to classify patients with HF.51 Beyond EF, LV global longitudinal strain (GLS) is recommended to further evaluate LV function and a cut-off <16% should be considered to support the diagnosis.52 Diastolic function parameters are also useful to identify patients with HF both at rest and during stress echocardiography and have been proposed as major criteria in the heart failure association pre-test assessment, echocardiography and natriuretic peptide, functional testing, final aetiology (HFA-PEFF) score.51 No differences in these parameters have been demonstrated between men and women in HFpEF cohorts, except for a higher peak A wave velocity and consequently a lower E/A ratio in women.50
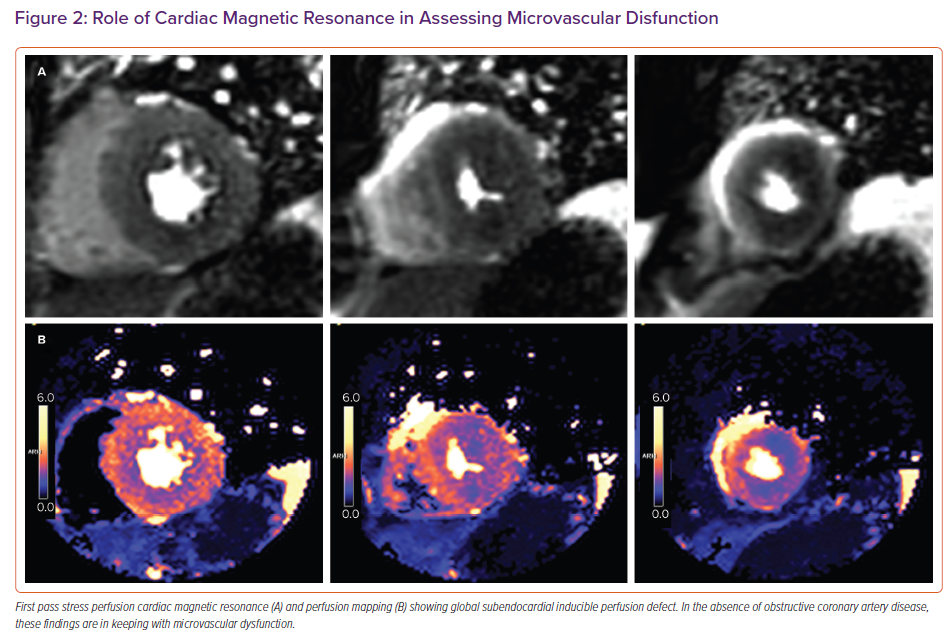
Although normal values do not exclude the diagnosis, LV geometry and mass should also be assessed using sex-specific cut-off values (LV mass index >95 g/m2 in women and >115 g/m2 in men.53 Finally, an LA volume index >34 ml/m2 has been used as a marker of high LV filling pressure in the absence of other alternative causes, whether Doppler assessment of tricuspid regurgitation (TR) peak velocity and estimated pulmonary artery systolic pressure is suggested to identify pulmonary hypertension.54,55 CMR should be used in suspected HFpEF patients with suggestive symptoms but inconclusive echocardiography. CMR tissue characterisation allows detection of myocardial fibrosis, deemed to be closely related to LV diastolic dysfunction. In fact, assessment of CMR-T1 extracellular volume (ECV) fraction independently predicts invasively measured LV stiffness in HFpEF and could be useful in a further pathophysiological classification of these patients.56 Presence of LGE has been described in more than a third of HFmrEF and HFpEF patients and has been associated with a worse prognosis.57 In addition, quantitative assessment of global myocardial perfusion reserve (MPR) by CMR could detect coronary microvascular dysfunction, described in most patients with HFpEF, especially women (Figure 2).58 Finally, the role of nuclear imaging in HFpEF is limited to the work-up of cardiac amyloidosis with (99 m)Tc-3,3-diphosphono-1,2-propanodicarboxylic acid (Tc-DPD) scintigraphy. Cardiac amyloidosis can be overlooked but it is estimated to be the cause ~10% of HFpEF in older patients.59
Breast Cancer-related Chemotherapy-induced Cardiomyopathy
Although breast cancer therapy with anthracyclines and trastuzumab has improved survival rates, it has been associated with an increased risk of developing HF and cardiomyopathy and CVD is the main cause of death in this patient group.60,61 Early identification and prompt initiation of therapy for anthracycline-related cardiotoxicity can prevent LV dysfunction and promote the recovery of LV systolic function.49,62 Therefore, cardiac monitoring of women undergoing chemotherapy for breast cancer is extremely important in these clinical scenarios. In particular, accurate LVEF monitoring is strongly recommended for monitoring trastuzumab-related toxicity, which is defined by an absolute LVEF decline of ≥16% from pre-treatment values or an LVEF decrease ≥10% from pre-treatment values to below the lower level of normal as defined by the institutional laboratory.63 In line with American Society of Echocardiography (ASE) and European Association of Cardiovascular Imaging (EACVI) recommendations, a decline in LVEF of >10% points to a value <53% (normal reference value for 2D TTE) is defined as cancer therapeutics-related cardiac dysfunction.64 TTE is the first-line imaging modality to assess myocardial, valvular or pericardial complications related to chemotherapy.64
The assessment of LVEF by the new TTE techniques (real-time 3D TTE) is well correlated with CMR or nuclear ventriculography at baseline, 6 and 12 months after initiation of chemotherapy.65,66 Reduction in deformation indices assessed by 2D speckle tracking echocardiography (STE) precedes the decrease in LVEF during chemotherapy and can be used as a marker of early cardiac injury. There is evidence that treatment with anthracyclines is associated with early reduction in global longitudinal, circumferential, radial strain or systolic or diastolic strain rate.67–69 In a multicentre study, 81 women treated with anthracyclines followed by taxanes and trastuzumab were assessed by echocardiography every 3 months during their chemotherapy.67 Peak longitudinal strain <19% at the completion of the anthracyclines treatment detected subsequent cardiotoxicity (sensitivity 74%, specificity 73%), while neither radial nor circumferential strain had predictive value.58 Reductions of GLS >15% from baseline are clinically significant in line with the ASE/EACVI consensus statement.64 It should be noted that the use of myocardial contrast agents might be useful in patients with breast cancer after mastectomy and chest irradiation due to poor delineation of the endocardial border. However, the use of contrast-enhanced 3D echocardiography is not recommended in clinical practice for the accurate measurements of the LV volumes and EF.64,70
There is evidence that 2D TTE had a sensitivity of 25% and a false-negative rate of 75% for detection of EF <50%, while 3D TTE had 53% and 47%, respectively compared with the CMR determined EF cut off value of <50%.71 CMR is indicated for the LVEF measurement when TTE images are suboptimal or TTE examination is not well tolerated in patients with breast cancer due to pain in the post-surgical site.6 Late LGE CMR can detect early trastuzumab-induced cardiotoxicity identifying sub-epicardial linear LGE pattern in the myocardium.72 Moreover, stress perfusion CMR plays an important role in excluding myocardial ischaemia as a contributing factor in LV dysfunction in the diagnostic work-up for chemotherapy-induced cardiomyopathy.6 Furthermore, there is evidence that the anthracycline-induced reduction in LV mass detected by CMR is associated with worsening HF symptoms independent of EF.73 The application of novel CMR techniques including ECV and T1 mapping holds promise in the follow-up of patients receiving therapy with anthracyclines. One study has demonstrated that native T1 with elevated pre- and post-treatment with anthracyclines (1,058 ±7 ms and 1,040 ±7 ms, respectively) versus comparators (965 ±3 ms, p<0.0001 for both comparisons) and ECV values were higher in anthracycline-treated patients (30.4 ±0.7%) compared with pre-treatment (27.8 ±0.7%, p<0.01) or cancer-free controls (26.9 ±0.2%, p<0.0001).74
Takotsubo Syndrome
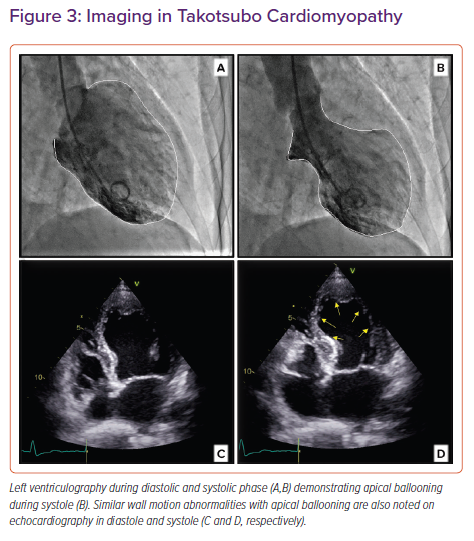
Takotsubo syndrome (TTS) is an acute condition characterised by a reversible LV dysfunction, usually involving the apical segments and triggered by emotional or physical stress (Figure 3).75 Nearly 90% of TTS patients are white, post-menopausal women.76 The clinical presentation often mimics an acute coronary syndrome (ACS), and the most frequent observed complication is acute HF, described in 12–45% of TTS patients.77 Coronary angiography is therefore often required to exclude obstructive CAD according to guidelines. The left ventriculography could also reveal the ‘apical nipple sign’ — a small, very apical area of preserved contractility in the context of ‘apical ballooning’ — observed in 30% of TTS cases but not in ACS patients.78 Cardiac CT angiography is a non-invasive alternative to coronary angiography in patients with low pre-test probability of CAD, or suspected of recurrent TTS, or with life-threatening comorbidities, such as stroke, intracranial haemorrhages or sepsis. Echocardiography has a key role in diagnosis and follow-up of TTS. The assessment of LV wall motion abnormalities (WMAs) allows the typical apical form to be distinguished from atypical (mid-ventricular, basal or even focal) and a wall motion score index ≥1.75 could help to differentiate TTS from anterior MI.79 The pulsed-wave Doppler interrogation is mandatory to exclude the concomitant presence of LV outflow tract obstruction, demonstrated in up to 25% of patients.80 The acute changes in LV geometry could also cause mitral regurgitation (MR), observed in 14–25% of TTS cases.81 The assessment of E/e’ ratio, LV function and moderate-to-severe MR is particularly important as they have been associated with adverse in-hospital outcomes.82 RV free wall strain should also be used instead of conventional parameters to assess RV involvement, described in about a third of cases and independently associated with poorer prognosis.83,84 CMR is recommended for further evaluation and diagnosis of TTS patients. The absence of LGE at high threshold (defined as signal intensity 5 SD above the mean of remote myocardium) is in fact typical of TTS, despite some low-threshold patchy LGE that is occasionally observed.85 Detection of myocardial oedema is possible using a ≥1.9 ratio between the T2-weighted signal intensity of the myocardium and the skeletal muscle.86
Nuclear imaging is not routinely used in TTS; however, an abnormal glucose metabolism and an impaired metaiodobenzylguanidine uptake last even after complete recovery of WMAs and could therefore be used to diagnose a previous episode of TTS within months.87
Heart Failure in Systemic Disease
Women account for 80% of patients affected by autoimmune systemic disorders.88 The mechanisms underlying the augmented cardiovascular risk observed in this population are several and include, among others, accelerated atherosclerosis, thromboembolism, vasculitis and HF, mainly with preserved EF.89 Echocardiography is the first imaging modality used in the cardiac assessment and follow-up of these patients, evaluating diastolic and systolic function and early LV remodelling. However, tissue characterisation by CMR could identify early cardiac inflammation/oedema using either T2-weighted imaging or T2-mapping. Moreover, myocardial oedema, hyperaemia and fibrosis could also be detected by a T1-based method, such as T1-mapping, ECV and/or T1-weighted LGE.90 Of note, several patterns of LGE – diffuse subendocardial, subepicardial and even transmural – have been described even in patients with normal echocardiography.91 Moreover, stress perfusion CMR with qualitative and quantitative MPR assessment using CMR to help differentiate underlying macro and microvascular complications in women often unable to exercise due to musculoskeletal compromise.92 For the same reason, coronary CTA could be used to rule out CAD especially in patients with low pre-test probability.93
Patients with thalassemia major are at increased risk of LV dysfunction due to myocardial iron overload (MIO). Even when men and women exhibit similar rates of MIO, women demonstrate about 50% lower risk of cardiac involvement possibly related to their relative resistance to chronic oxidative stress. Due to this evidence, a cardiological assessment every 2 years (instead of 1 year) has been proposed for women older than 20 years including ECG, Holter ECG, echocardiography and CMR.94 Advanced imaging techniques could be particularly relevant in this population to detect subclinical LV dysfunction. In fact, GLS ≤-17%, lower peak twist and peak apical rotation values and reduced circumferential strain have demonstrated good correlation with MIO.95 However, the gold standard technique to quantify MIO is T2* technique by CMR that is highly reproducible and has been demonstrated to predict the need of iron chelation therapy.96
Peripartum Cardiomyopathy
Peripartum cardiomyopathy (PPCM) is a heart muscle disease with its onset during pregnancy or in the 6 months following miscarriage or delivery. It is characterised by LV systolic dysfunction (EF <45%) and presents as HF for which no other aetiology is identified.97 Many potential causes of PPCM have been proposed, including myocarditis, nutritional deficiencies, angiogenic imbalance, autoimmunity, inflammation and a pathological response to haemodynamic stress.98 The oxidative stress-mediated cleavage of the hormone prolactin into a cardiotoxic fragment (16-kDa prolactin) induces endothelial damage and impairs cardiomyocyte metabolism and is a driver of PPCM.99 There is significant genetic overlap with DCM, with about 15% of patients carrying truncating variants in DCM-associated genes, mainly in the TTN gene.100
The incidence is approximately 1:1,000 pregnancies but this varies significantly by region.98,101 These are likely to be underestimated as many PPCM presentations with symptoms such as breathlessness and fatigue are misinterpreted as non-specific symptoms of pregnancy.102 Major adverse events, including cardiac transplantation and mechanical circulatory support (2–7%), thromboembolism and ventricular arrhythmias occur in about 13.5% of patients.100,103 Mortality estimates range from 2–12.6%.104–106 Echocardiography allows diagnosis and differentiation between other pregnancy-related cardiac conditions. LVEF is usually reduced and GLS seems promising in identifying early remodelling in PPCM.107 Moreover, echocardiographic assessment of LV end-diastolic diameter and LVEF could allow a further prognostic stratification. CMR could provide information regarding LVEF, volumes, RV involvement, myocardial oedema and fibrosis. Non-ischaemic LGE has been found in up to 40% of PPCM patients and is associated with a worse prognosis.108 Moreover, CMR could be useful in the diagnosis of thromboembolism when echocardiography is not conclusive.
Pulmonary Arterial Hypertension and Right Heart Failure
Pulmonary arterial hypertension (PAH) is more common in women, particularly in patients with PAH secondary to systemic sclerosis, where there is high predominance in women.109
TTE is the recommended first-line non-invasive technique for the diagnostic work up of suspected PAH.110 It enables the assessment of RV function by multiple parameters including tricuspid annular plane systolic excursion (TAPSE), the systolic velocity of tricuspid annulus (S’), fractional area change, RV myocardial performance (Tei index), RV dP/dt, RVEF and RV strain.111 TTE also enables the measurement of pulmonary artery systolic pressure (PASP) from maximum transtricuspid valvular regurgitant jet velocity obtained by Doppler in addition to right atrial pressure and there are high correlations between echocardiographic Doppler imaging and invasive measurements of PASP.112,113 However, poor acoustic window, the misalignment with the tricuspid regurgitant jet and the failure in the modified Bernoulli’s equation’s assumptions are possible limitations resulting in inaccurate PA measurements, causing discordance between non-invasive and invasive measurements.114 In addition, many echo parameters such as the presence of pericardial effusion, the right atrial area, TAPSE and eccentricity index are useful in the risk stratification of PAH patients are proven to be related to survival in patients with pulmonary hypertension.114 Importantly, TTE enables monitoring after pulmonary endarterectomy and assessment of response to medical treatment for PAH.115,116 3D TTE can provide accurate evaluation of RV function avoiding the geometric assumptions that are intrinsic to 2D TTE.117 A study has demonstrated that 3D TTE and 2D STE parameters are corelated with RV HF haemodynamics better than the conventional echo variables, although 3D TTE for the assessment of RV function has not yet been applied in routine clinical practice.118
CMR studies have shown that women with PAH have better RV function than men and respond better to medical treatment for PAH compared to men.119,120 CMR is a reliable non-invasive imaging technique for the assessment of cardiac structure and function and the risk stratification of PAH patients. In particular, CMR can evaluate the RV function and ventricular septal motion abnormalities accurately.121 It is established that LGE at the RV insertion points on the interventricular septum is a marker of more advanced disease and poor prognosis.122–124 Another study showed that increased right atrial volume is associated with lower transplantation-free survival in patients with precapillary PAH (HR 2.1; 95% CI [1.1–4.0]).125
Several CMR parameters have been associated with prognosis in patients with PAH. A study showed that elevated RV end-diastolic volume is the most accurate marker for progressive RV failure and reduced RV stroke volume is associated with increased mortality.126,127 The EURO-MR study showed that the evaluation of RV function by CMR can be used to assess the clinical benefits in patients receiving PAH-targeted therapy (endothelin receptor antagonists or phosphodiesterase type-5 inhibitors).128
Conclusion
HF in women composes a clinical syndrome with unique characteristics and clinicians should be aware of the sex-specific differences when using multimodality cardiac imaging.
Moreover, attention should be paid to specific strengths and weaknesses of each imaging modality, especially when assessing the diagnosis, prognosis and management of pregnant (or potentially pregnant) patients. All this evidence, coupled with the under-representation of women in clinical trials, points to the need for more scientific data supporting the management of women in HF in clinical practice.