Cardiogenic shock (CS) is a circulatory failure as a consequence of left, right or biventricular dysfunction.1 It leads to critical end-organ hypoperfusion due to primary cardiac dysfunction.1 Therefore, CS is not only a cardiac disease but also a multiorgan dysfunction syndrome involving the entire circulatory system, often complicated by a systemic inflammatory response syndrome.2 The goals of haemodynamic support for patients with CS should be circulatory support, ventricular unloading/support, coronary arteries perfusion and decongestion.3
Unfortunately, pharmacological approaches fail to achieve all the objectives.3 Often drug therapy will solve only one element, but this is at the cost of another.3 For example, although vasopressors sustain haemodynamic status by increasing mean arterial pressure, their use can impair microvascular organ perfusion, increase left ventricular afterload and myocardial work and cause myocardial ischaemia.3 Therefore, in recent decades, more aggressive strategies, such as temporary mechanical circulatory support (TCS), have been investigated to address all the elements to achieve an optimal haemodynamic status.
TCS includes a group of devices used generally for less than 30 days to maintain adequate organ perfusion (Table 1).4 TCS counteracts acute circulatory failure, which might also arise after cardiac surgery.4 Moreover, since it was introduced, TCS has been used as a bridge to a more definitive therapy.5 The management of CS with TCS has advanced in the past decade.5 The scope of applications has widened, and easily deployable devices are significantly more available.5 High-risk procedures, for example, percutaneous coronary interventions (PCI) and ventricular tachycardia ablation, have also started to involve TCS device use.5
However, indications for TCS and device selection are part of a complex process requiring consideration of the severity of CS, early and prompt haemodynamic resuscitation, specific patient risk factors, technical limitations, adequate resources and training and assessment of the futility of care.6 Early intervention with the most appropriate mechanical circulatory support device may improve outcomes.6 The aim of this review is to provide an overview of the TCS devices currently available for patients with CS.
Temporary Mechanical Circulatory Support Devices
The technical features of percutaneous assist devices available are compared in Table 2.
Intra-aortic Balloon Pump
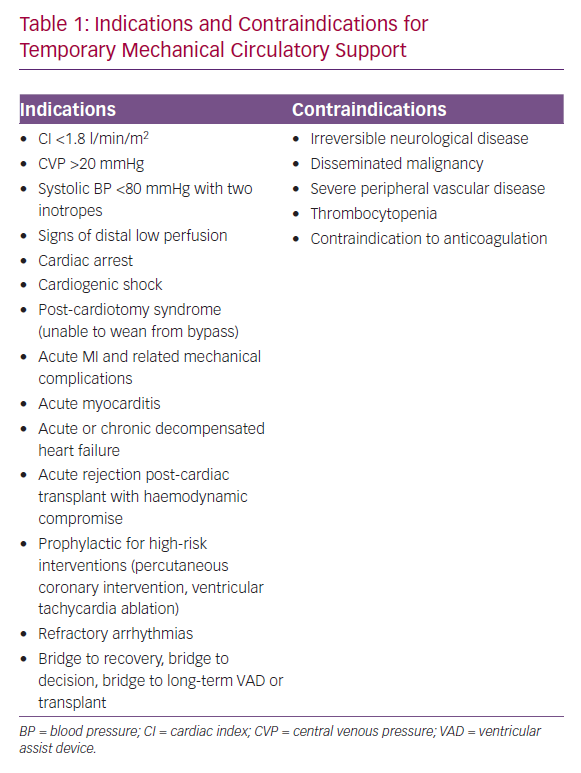
The intra-aortic balloon pump (IABP) is the most frequently used form of TCS (Figure 1A).7 Straightforward insertion, ready availability and low cost have made IAPB use common in the treatment of the acute heart failure.7 When positioned in a timely manner, it can play a critical role in the rescue of patients with acute ischaemic MI.7
However, the benefit of IABP therapy is still being debated, and a considerable gap exists between current guidelines and clinical practice. The Intra-aortic Balloon Counterpulsation in Cardiogenic Shock II (IABP-SHOCK II) trial failed to show that an IABP can improve 30-day and 1-year mortality if used in conjunction with optimal medical therapy and early revascularisation.8,9 Accordingly, the European Society of Cardiology guidelines downgraded IABP use for cardiogenic shock from a class I to a class III B recommendation (Table 2).10–12 In the American Heart Association/American College of Cardiology guidelines, IABP use has been downgraded to a class IIb B recommendation based on registry data.13,14 However, the IABP-SHOCK II trial involved a selected population. Nearly half of the subjects enrolled in the study had experienced resuscitation before IABP implantation, which probably resulted in a poor neurological prognosis for many of them regardless of cardiac disease type, and almost all were supported by a high dose of catecholamine at implantation.8,9 Therefore, questions remain over the role of IABP.
Despite these negative results, IABPs may still benefit subgroups of patients.15 There is stronger evidence of the efficacy of IABPs in high-risk PCI, based on a prospective randomised trial that included 300 high-risk PCI patients with severe left ventricular dysfunction and extensive coronary disease.16 Moreover, a recent meta-analysis confirmed that the use of IABP in high-risk PCI caused a significant reduction in long-term, all-cause mortality.17 Furthermore, improved organ function and whole-body perfusion associated with reduced endothelial activation have been shown when IABP is used during cardiopulmonary bypass for coronary artery bypass grafting.18,19
Several studies have reported a significant benefit of combining IABP with venous-arterial extra-corporeal membrane oxygenation (VA ECMO); this configuration, theoretically, can reduce the left ventricular (LV) afterload.20–25 The association of the two devices may have a synergistic effect in the treatment of the acute cardiac failure.20–25 Recently, Wang et al. showed that patients receiving VA ECMO with IABP had greater success in weaning from VA ECMO.26 However, they did not have lower in-hospital mortality rates than those with VA ECMO but without IABP. Combined support devices also facilitated cardiac function recovery in post-cardiotomy patients.27–29 These findings underline that, despite apparent benefits from the combination of the two devices, further investigations are warranted to conclusively prove the actual role in cardiogenic shock patients requiring TCS.
iVAC
The IABP drive unit can also be used for another TCS, a pulsatile catheter pump called the iVAC (PulseCath, Figure 1B). The iVAC is a minimally invasive pneumatic circulatory assist device that offers circulatory support of 2.5–3.0 l/min, so is positioned between an IABP and a conventional ventricular assist device (VAD).28 It is implanted through the right axillary artery and it directly unloads the LV by active blood aspiration during systole while it creates pulsatile flow into the ascending aorta in diastole.28 Side-effects from its use are mainly haemolysis, which is usually evident within 2 days of implantation, and platelet consumption caused by shear stress and fragmentation, but both effects are minimal and comparable to side-effects of other pulsatile devices.29 However, the iVAC is easy to implant, and there is no need for additional equipment beyond a standard IABP driver unit and transoesophageal echocardiography apparatus.30
In a prospective pilot study of 14 patients, den Uil et al. reported that the iVAC offered support in high-risk PCI with 100% angiographic success.30 The PULsecath mechanicaL Support Evaluation (PULSE) study (NCT03200990) aims to evaluate iVAC and Impella CP (Abiomed) in patients with severe LV impairment undergoing PCI. However, further research addressing its role in the TCS family is required.
Temporary Ventricular Assist Devices
Impella
The Impella system (Figure 1C) is a miniaturised, continuous-flow, axial pump in a single pigtail catheter.31 Using the Archimedes screw principle, it pumps blood from the LV to the ascending aorta by rotating a screw-shaped surface inside a small, hollow pipe that traverses the aortic valve.31 It produces a non-pulsatile axial flow, pumping blood from the LV into the ascending aorta.31 The Impella 2.5 is the smallest device, generating up to 2.5 l/min of blood flow, and it is usually delivered via the femoral or axillary artery.31 The largest device in the same family can develop 5 l/min of blood flow, but requires a surgical cut-down, although a novel trans-caval approach has recently been designed.32,33 The Impella CP provides an intermediate level of support of 3.0–4.0 l/min.34 In addition, the Impella Right Percutaneous (Impella RP) is available for the treatment of right heart failure.34 The Impella RP, implanted through the femoral vein, provides right ventricular support. However, it could make the patient dependent on the sedation/paralysis status and the right ventricle filling. The variations of the blood volume filling the right ventricle and patient movement could affect the performance of the device.
Micro-axial flow pumps can rapidly reduce ventricular wall stress and myocardial oxygen consumption, increasing antegrade flow and consequently reduce ventricular pressure and volume.35 Higher coronary flow velocities and favourable microvascular resistance response with increasing Impella support levels have been described as well.36 Accordingly, in acute left ventricular failure, especially if caused by myocardial ischaemia, mechanical support with Impella confers several beneficial effects.35,36
A survival rate of 94% at 30 days has been reported by a multicentre, prospective feasibility study, including patients treated by the Impella 5.0 for postcardiotomy CS.37 Data from the USpella Registry similarly showed that, in patients with CS undergoing PCI, those receiving an Impella before a PCI procedure had lower mortality rates than those in whom the device was implanted only after revascularisation.38 Moreover, in a retrospective single-centre analysis comparing the outcomes of patients with CS who received the Impella 2.5 or 5.0, the Impella 5.0 group had better 30-day survival.39
Two small randomised controlled trials, studying the use of the Impella and IABP for patients with CS, have been published.40,41 In the Efficacy Study of LV Assist Device to Treat Patients with Cardiogenic Shock (ISAR-SHOCK) trial, patients with acute MI complicated by CS were randomised to receive the Impella 2.5 or an IABP.40 Compared to the IABP, the Impella group showed a higher cardiac index at 30 minutes after implantation, but no difference in mortality was observed.40 Similarly, in the IMPella versus IABP Reduces mortality in ST-elevation MI (STEMI) patients treated with primary PCI in Severe cardiogenic Shock (IMPRESS) trial, the Impella CP was not associated with better recovery of myocardial function or lower mortality when compared to IABP.41
The introduction of percutaneous micro-axial flow pumps also allowed retrograde trans-aortic unloading of the LV during VA ECMO, providing effective decompression of the left chambers with a less invasive approach than other surgical techniques.42 Animal models originally revealed that the Impella device could decrease LV end-diastolic pressure and pressure-volume area even more than ECMO in an acute failing heart, and its use was associated with a higher successful defibrillation rate.43 Accordingly, in a retrospective multicentre cohort of patients with CS treated with VA ECMO plus an Impella device, Pappalardo et al. reported that their combination resulted in lower in-hospital mortality and higher rates of bridging to recovery.44 Finally, Patel et al. recently showed that using an Impella device in addition to a VA ECMO for CS is associated with improved survival and reduced need for inotropic support, without higher complication rates.45
The use of intracardiac micro-axial flow pumps, although less invasive than surgically implanted devices for mechanical support, is not without potential complications. The most commonly reported are limb ischaemia, bleeding and vascular injury, ranging from haematoma, pseudo-aneurysm and arterial-venous fistula to retroperitoneal haemorrhage.46 Mechanical erythrocyte shearing often causes haemolysis as well, which has been observed within the first 24 hours of use in up to 10% of patients.46
The Impella device is positioned in the LV, across the aortic valve plane, so has obvious contraindications, including significant aortic valve disease, the presence of a mechanical aortic valve and LV thrombus.47 The device should not be placed in patients with severe peripheral arterial disease or those who cannot tolerate systemic anticoagulation.31 In patients with a known, pre-existing ventricular septal defect, the worsening of right-to-left shunting and hypoxaemia have to be taken into account as well.31 However, in spite of the contraindications and possible complications, since the device gained FDA approval in 2008, an increasing number of patients have been treated with Impella devices worldwide, and this has been supported by more favourable literature evidence.48
Some of the latest preclinical experiences have highlighted the key role of LV unloading, along with a 30-minute coronary reperfusion delay, in limiting ischaemia–reperfusion injury.49,50 This approach may promote myocardial recovery, enhance the activity of kinases, preserve mitochondrial integrity, increase the production of cardioprotective cytokines and favour microcirculatory coronary flow.49,50 In this context, Kapur et al. recently showed there was no difference in infarct size of patients affected by acute MI without CS, when comparing subjects treated traditionally by immediate PCI with those who received acute LV unloading with an Impella device, even before myocardial reperfusion.51 This pilot study may represent a turning point for the progress in cardioprotection against acute myocardial ischaemia, by claiming, for the first time, a delay in coronary reperfusion, and considering LV unloading in this setting as a true priority.
TandemHeart
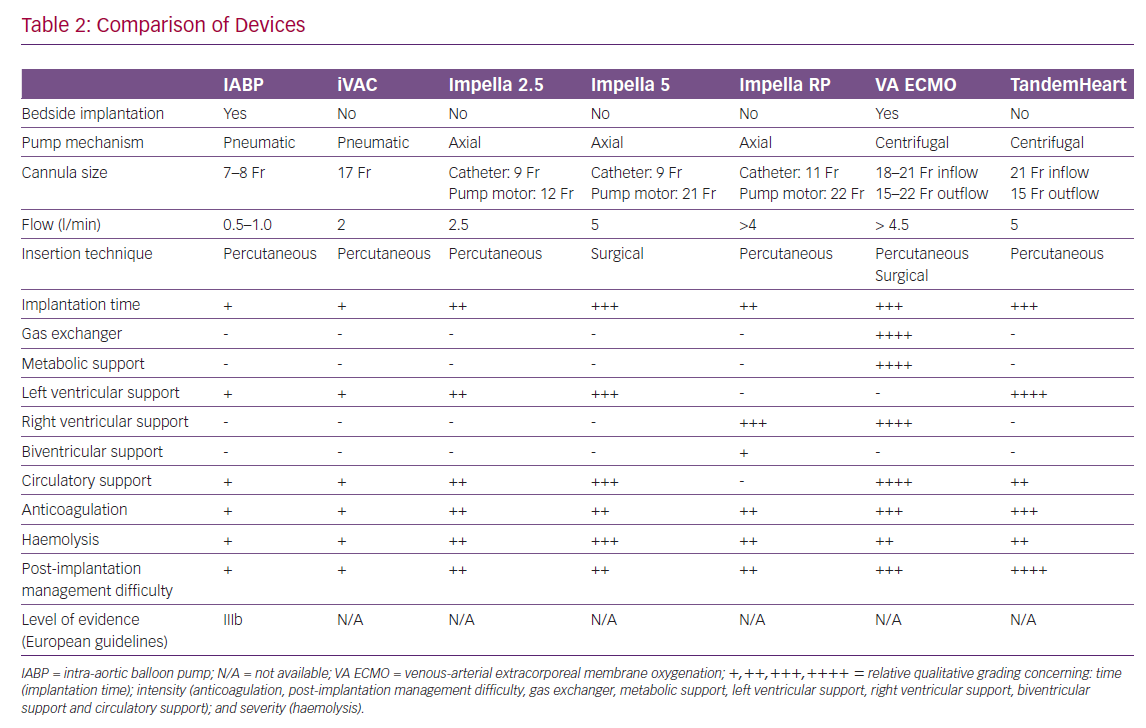
The TandemHeart (LivaNova) system (Figure 2B) is a percutaneous VAD with an extra-corporeal continuous-flow centrifugal pump (flow rates up to 4 l/min at a maximum speed of 7,500 rpm).52 It is a left atrium-femoral artery system.52 Oxygenated blood is withdrawn from the left atrium, which is accessed using a standard trans-septal technique and pumped into the systemic circulation in the femoral artery, thereby bypassing the left heart.52 Right ventricular support can be achieved by placing the inflow cannula in the right atrium and the outflow cannula in the pulmonary artery.52 The need for trans-septal puncture for LV support is a potential limitation to its widespread use.52 Specific contraindications are ventricular septal defect (this could cause right to left shunting and hypoxaemia) and aortic insufficiency.52 The complications are limb ischaemia, tamponade after a transeptal puncture, bleeding, infection and thromboembolism.53,54 Several studies demonstrate an increase in cardiac index and mean arterial pressure with a consequent decrease in pulmonary capillary wedge pressure.55–60 In 2005, Thiele et al. reported their experience with TandemHeart support in patients with CS after acute MI.58 Patients were randomised to haemodynamic support with either an IABP or the TandemHeart.58 Those fitted with the device showed a statistically significant improvement in cardiac output, cardiac power index, pulmonary capillary wedge pressure, mean pulmonary arterial pressure, central venous pressure and serum lactate level.58 On the other hand, there was an increased risk of limb ischaemia and coagulopathy.58 There was no difference in mortality.58
ProtekDuo
The ProtekDuo (LivaNova) is a dual-lumen cannula, placed through the internal jugular vein.61 One lumen is for the inflow, draining the right atrium; the second lumen serves as outflow, perfusing the pulmonary artery.61 This percutaneous device was designed specifically for right ventricular support.61 It provides a minimally invasive option because of easy insertion and removal.61 Moreover, the patient can be mobilised.61 Furthermore, an oxygenator can be inserted into the circuit (an ambulatory oxygenator right ventricular assist device or OxyRVAD), which contributes to systemic oxygenation.62
Veno-arterial Extra-corporeal Membrane Oxygenation
VA ECMO (Figure 2A) is a form of temporary mechanical circulatory and simultaneous extra-corporeal gas exchange for acute cardiorespiratory failure.63 All VA ECMO circuits consist of a venous (inflow) cannula, a pump, an oxygenator and an arterial (outflow) cannula.63 During ECMO support, deoxygenated blood is drained from the venous circulation, passes through the pump into the oxygenator, where gas exchange occurs, and is then returned oxygenated to the arterial circulation.63 Patients may be cannulated centrally or peripherally.63 Peripheral VA ECMO is commonly applied via the femoral artery and vein, either surgically or percutaneously using the Seldinger technique.63 Central VA ECMO is primarily implemented in the operating room and cannulas are usually secured directly to the large vessels or heart chambers while the chest is open.63 Left atrial VA ECMO involves the trans-septal placement of a venous femoral cannula to simultaneously drain both atria in patients with severe left ventricular systolic dysfunction.64 Left atrial VA ECMO allows the drainage of both atria and decreases pulmonary oedema in patients with severe heart failure.64
In recent years, VA ECMO has become the firstline therapy in the setting of CS unresponsive to standard therapy, since it provides both respiratory and cardiac support, is easy to insert and can stay in place for several days as a bridge to making a decision, which could be for recovery, transplantation or long-term mechanical support.65–68 However, despite advances in technology and cannulation strategies over time, the prognosis of patients in refractory CS supported with ECMO remains poor.69 A previous retrospective analysis by Sheu et al., reporting data on 30-day survival in 46 patients with STEMI in profound CS, found a morality rate of 39.1%.70 Furthermore, in the cohort reported by Belle et al., in-hospital mortality was 51.9% in 27 patients with CS.71
As physicians care for an ever-increasing number of patients with refractory CS, we should better understand which patients could benefit from VA ECMO. Patient selection for VA ECMO must take into account the underlying diagnosis, comorbidities and whether there is a viable exit strategy, such as recovery, heart transplantation (HTx) or long-term support.68 Several studies have highlighted the importance of the underlying diagnosis in determining survival. Patients with potentially reversible causes of myocardial injury, such as acute myocarditis, have better survival rates those patients with CS after acute MI or cardiac surgery.67,72,73 In addition to considering the underlying diagnosis, given the importance of multiorgan dysfunction syndrome in determining clinical outcome, the timing of VA ECMO initiation is key. Just as premature use may expose a patient to undue risks and complications, delayed initiation may be medically futile. The ideal window for deployment is after other, less invasive treatments have been considered or exhausted, but before the onset of significant end-organ dysfunction.63
Peripheral VA ECMO is a potential option for a refractory cardiogenic shock because it quickly improves haemodynamics, can be initiated outside the operating room and requires a relatively non-invasive procedure.63 However, since it provides retrograde blood flow in the aorta, it may increase LV afterload, leading to an increase in LV pressure and wall stress, which impair myocardial recovery and may ultimately delay cardiac contractility improvement.74 Based on these adverse mechanisms, it is clear that unloading the LV during VA ECMO may provide an actual LV functional rest or reduce complications due to counterflow generated by the temporary mechanical support.75 A variety of LV unloading strategies can be used after peripheral VA ECMO has been started, such as IABP, atrial septostomy, pulmonary artery drainage and percutaneous trans-aortic ventricular assist device implantation.75
Despite the well-known controversy, IABP remains widely used in combination with VA ECMO.75 Percutaneous approaches using unloading devices, such as Impella equipment, are becoming increasingly used.75 However, the optimal strategy to achieve LV decompression remains unclear.75 Peripheral VA ECMO is also a viable alternative for right ventricular failure caused by a primary dysfunction or as a consequence of a pulmonary disease.63 It can be applied percutaneously at the bedside and the most common configuration is femo-femoral.63 In select cases, it is possible to add a venous cannula in the pulmonary artery to drain the right atrium from two places (VV-VA ECMO).80 These settings increase LV afterload and systemic mean arterial pressure.76 If left ventricular function is impaired, it is often useful to add a second device to decompress the LV.76
Timing of weaning from ECMO is important to achieve good outcomes from this therapy. At the crux of the decision to wean patients off support is whether adequate myocardial recovery to provide sufficient blood and oxygen delivery to organs to meet metabolic demands has been demonstrated.63 Although there is controversy over the degree of acceptable pharmacological support, data suggest that lower levels of inotropes at the time of weaning are associated with better outcomes.77 If cardiac recovery is unlikely or cannot be achieved despite medical therapy optimisation and recovery of end-organ function, HTx or long-term mechanical support should be considered.78
Decision Management
If there are signs of cardiac recovery and sustained optimal peripheral perfusion (such as improved cardiac indices, left ventricular ejection fraction, tricuspid annular plane systolic excursion >12 mm, low lactate levels or low wedge pressure), the patient should be weaned from TCS. However, if there are no signs of improvement in haemodynamic parameters or clinical signs, then durable mechanical circulatory support or the withdrawal of TCS (for patients with permanent neurological damage) should be considered.
In recent years, a shortage of donors and long waiting-list times resulted in a progressive increase of the number of patients who are bridged to HTx under mechanical circulatory support.79 A potential alternative, in suitable candidates, would be transition from TCS to a durable LVAD. This condition has been shown to be characterised by acceptable survival.80 A recent meta‐analysis found favourable survival to discharge rates in such patients, with 19% of patients undergoing TCS transitioned to durable LVAD with in-hospital survival ranging from 60% to 100%.81 Similar results were reported by Barge‐Caballero et al. in their retrospective study.82 Patients on durable mechanical circulatory support can subsequently be electively evaluated for transplantation.
Outcomes after transplantation in patients on long‐term, continuous‐flow LVADs are now similar to those achieved in elective patients transplanted without bridging with mechanical circulatory support.83 The use of mechanical circulatory support bridging to HTx has several advantages. Most importantly, the use of donor organs is minimised. Furthermore, for some patients, remaining on LVAD as a destination therapy may turn out to be preferable to transitioning to HTx, either because of concomitant medical issues or simply because of patient preference. Finally, some patients may experience cardiac recovery that means the device can be removed and a donor organ is not required after long‐term LVAD support.84 Disadvantages of the bridge-to-bridge approach include the need for repeated surgery and consequent increase in costs. Moreover, the strategy cannot be used in all cases. Transition to a durable LVAD requires adequate right ventricular function to sustain satisfactory circulatory function on the LVAD.
TCS as a technology is increasingly being used to maintain or even replace basic biological functions. However, timely TCS withdrawal should be considered if the chances of recovery are extremely poor or if severe complications (mainly neurological) occur. This challenging situation has raised conflicting behaviours among doctors. Recently, some physicians have been increasingly engaged with families in shared decision-making.85 Others think that physicians should have the right to discontinue the TCS over a family’s objection.85 TCS devices increase our ability to push the limits of life and delay death (even if only temporarily), bringing potential ethical complications. Further research and debate are warranted where solutions to this complex ethical dilemma can be propose and agreed upon.
Conclusion
Major technological evolution has enabled TCS to take on a larger role in the treatment of acute CS over the last decades. Physicians and surgeons are now equipped with an assortment of TCS devices. These are being used both to treat and prevent circulatory collapse. Moreover, they improve haemodynamics in a large array of clinical situations. The choice of adequate TCS is typically guided by the availability of devices and patient-specific factors and conditions. Given that studies that directly compare TCS devices are lacking, further research is needed to provide better guidance on device selection and placement.