The incidence of heart failure (HF) has remained stable over recent decades. However, the prevalence of HF is on the rise, presumably as a result of the progress made in its management with the introduction of several life-saving medical and device therapies.1 The overall aging of the population – together with the cumulative burden of predisposing conditions and comorbidities – also contributes to the growing prevalence of HF. After the age of 65 years, there is a twofold increase in the prevalence of HF in men and a threefold increase in women.2 Rates of all-cause mortality, all-cause hospitalisation and HF hospitalisation significantly increase with advancing age in both sexes.3,4 Aging is associated with a higher risk of morbidity and mortality because of a greater impact of comorbidities, higher risks of complications, and possible underuse of guideline-directed treatments (GDT). The latter is likely the result of difficulties imposed by polypharmacy, frailty, cognitive impairment, poor tolerance and adherence, along with limited social support that ultimately hinder the quality of care.5
The term ‘elderly’ has up until recently been applied to patients aged over 65 years, but with the population becoming older, this limit has shifted to over 70–80 years. The HF population aged over 75 years has been largely underrepresented in randomised clinical trials (RCTs) assessing therapies for HF with reduced ejection fraction (HFrEF). Elderly patients typically comprise approximately 30% of participants in trials, and individuals with the severe or advanced comorbidities frequently observed in the real-world elderly patients are excluded.6,7 Although the majority of trials have not demonstrated heterogeneity in the efficacy or safety of treatments in different age groups, there remains uncertainty about tolerability, dosing and the risk–benefit ratio in older patients.8 This can make decisions about initiation or up-titration of GDT in elderly HF patients challenging. The purpose of this review is to provide a summary of evidence from clinical trials and real-world registries regarding the medical treatment of HFrEF in the elderly population.
Potential Reasons for Underuse of Guideline-directed Treatment in the Elderly
There are several reasons for the underuse and/or under-dosing of GDT in the elderly. These can be broadly grouped into the following categories: patient-associated factors, treatment-related aspects and healthcare system characteristics. Patient-associated factors of particular concern for the elderly include lower blood pressure, lower heart rate and lower BMI, greater severity of HF, and the burden of multiple comorbidities, frailty, cognitive impairment, polypharmacy and limited social support.9,10 Treatment-related aspects, such as poor tolerability, contraindications and adverse effects are also more commonly encountered in elderly and frail patients.11 Healthcare system characteristics that may adversely impact on GDT prescription include regional and international differences in healthcare system organisation, service availability and quality of care. A recent Heart Failure Association Atlas survey on the epidemiology of HF and resources for its management showed significant differences in reimbursement of standard HF medications and disparities in the availability of specialised centres for the multidisciplinary HF management in the European Society of Cardiology countries.12 These factors may have critical impact on the provision of HF medications and the availability of follow-up by cardiologists or HF specialists, who may be more experienced and confident to engage in GDT optimisation in the elderly compared with general practitioners, geriatricians or internal medicine specialists.13
Evidence from Clinical Trials
Patient Characteristics
Accumulating data suggest that elderly patients have distinct clinical characteristics compared with younger participants of RCTs. Elderly patients are more often female and tend to have more comorbidities, including coronary artery disease, hypertension, AF and chronic kidney disease, as well as higher baseline natriuretic peptide levels despite higher average left ventricular ejection fraction.14–16 They also tend to have a worse prognosis, but it seems that the mortality gradient across the age span has become less steep in the most recent clinical trials compared with earlier studies, most likely reflecting evolving benefits of more comprehensive contemporary care. However, even the latest trials demonstrate notable differences in background medical therapies between younger and the older participants, with the older participants being less likely to receive established disease-modifying drug therapies for HFrEF. There is also a tendency for older patients to obtain lower doses of study medications that require up-titration (Supplementary Material Table 1).14–16
Drug Therapies
β-blockers
Age-stratified analyses of data from RCTs on the efficacy and safety of β-blockers are sparse because of the underrepresentation of the oldest patients. A post hoc analysis of MERIT-HF participants aged ≥65 years showed a 37% reduction in all-cause mortality (RR 0.63; 95% CI [0.48–0.83]) among patients treated with metoprolol succinate, with a trend toward benefit in patients aged ≥75 years (RR 0.71; 95% CI [0.42–1.19]).17 The rates of adverse events (bronchospasm, depression and dizziness) that would be the cause of drug discontinuation were not higher in the elderly.18
The SENIORS trial has specifically addressed the efficacy and safety of the β-blocker nebivolol in the treatment of individuals with HF aged ≥70 years.8 The study showed a significant reduction in the combined outcome of all-cause mortality and cardiovascular (CV) hospitalisation in the nebivolol arm (HR 0.86; 95% CI [0.74–0.99]) but without a significant effect on all-cause mortality (HR 0.88; 95% CI [0.71–1.08]).8
A meta-analysis of the major trials with β-blockers including 13,833 HFrEF patients in sinus rhythm (median age 64, interquartile range 55–71) demonstrated a 24% risk reduction in all-cause mortality with β-blockers and an absolute risk reduction of 4.3% (number needed to treat 23; 95% CI [18–32]).19 β-blockers were superior in comparison to placebo across the range of age groups (p for interaction=0.1). There was also a reduction in the risk of hospitalisation for HF, although this effect was slightly attenuated at older ages (p for interaction=0.05). Likewise, there was an attenuation of the effect on CV mortality with aging (p for interaction=0.04), although there remained a trend toward a reduction in event rates even in the oldest patient group. Drug discontinuation rates were comparable regardless of age (14.4% in those receiving a β-blocker and 15.6% in those receiving placebo).
Post hoc analyses of clinical trials with β-blockers demonstrate that up-titration to the target doses may not provide incremental benefit over the mid-range doses. In the SENIORS trial, attaining 50% of the target dose of nebivolol had a similar impact on outcome as the target dose of 10 mg daily.20 However, patients in a low-dose group (1.25–2.5 mg daily) and those unable to tolerate any dose of nebivolol had an increased risk of death or CV hospitalisation.20 The MERIT-HF trial also showed similar reduction in total mortality with low (≤100 mg daily) or high-dose (>100 mg daily) metoprolol compared with placebo, which may be explained by a similar reduction in the heart rate.21 This notion is further supported by the CIBIS-ELD study, which demonstrated that the achieved heart rate after up-titration, rather than the dose of bisoprolol, was a significant predictor of lower mortality.22
Angiotensin-converting Enzyme Inhibitors and Angiotensin Receptor Blockers
Despite strong evidence about the benefits of angiotensin converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB) in the general population of patients with HFrEF, without evidence of age-related heterogeneity in major RCTs, none of the trials has specifically enrolled only older individuals and therefore data are limited in patients aged >75 years.23
A meta-analysis of five RCTs with ACEIs in patients with ischaemic aetiology of HF or left ventricular systolic dysfunction has documented a significantly lower risk of mortality (OR 0.74; 95% CI [0.66–0.83]), as well as lower risk of the composite endpoint of death, HF hospitalisation or MI in patients treated with ACEIs. Importantly, there was a nonsignificant age-by-treatment interaction for both outcomes (p=0.47 and p=0.95, respectively).24 In another meta-analysis of RCTs with ACEI in HFrEF, total mortality and hospitalisation for worsening HF were significantly reduced with ACEI treatment, with an OR of 0.72 (95% CI [0.59–0.89]) in patients aged <60 years and a favourable trend in those aged >60 years (OR 0.94; 95% CI [0.78–1.13]).25
A subgroup analysis of the CHARM-Overall trial has also reported a significant mortality benefit with candesartan in patients aged 65–75 years as well as in those aged >75 years, with a nonsignificant age-by-treatment interaction (p=0.26).26 Another sub-analysis of the CHARM programme assessed the efficacy of candesartan treatment across five age groups: <50 years (8% of all study patients), 50–59 years (19%), 60–69 years (31%), 70–79 years (33%), and ≥80 years (9%).14 The risk of CV death or HF hospitalisation increased from 24% in the youngest age group to 46% in the oldest age group, and there was a gradient in the risk of death (from 13% to 42%) across the age span. Relative risk reduction (15% in the overall study population) in CV death or HF hospitalisation with candesartan was similar regardless of age. Because of the higher morbidity and mortality in the elderly, the benefit increased with advancing age (event-rate reduction 3.8/100 treated patients in the youngest age group compared with 6.8/100 treated patients in the oldest age group). Of note, adverse events leading to drug discontinuation (hyperkalaemia, increase in serum creatinine and hypotension) occurred more frequently in the older age categories. However, the relative increment in the risk of adverse events with candesartan compared with placebo was similar regardless of age, except for an increase in serum creatinine, which was less frequent with candesartan in the elderly.14
A post hoc analysis of Val-HeFT, in which almost 50% of patients were aged >65 years, has demonstrated similar risk reduction in the co-primary endpoint of the first morbid event (death, sudden death, HF hospitalisation or urgent HF treatment) regardless of age.27 Accordingly, there was an 11.8% risk reduction (p=0.07) in morbidity in patients aged >65 years and a 14.6% risk reduction in those aged <65 (p=0.09). In addition, valsartan also had a beneficial effect on left ventricular function and size, quality of life and levels of natriuretic peptides, regardless of age.
Mineralocorticoid Receptor Antagonists
In the three pivotal RCTs of mineralocorticoid receptor antagonists (MRAs) in patients with HFrEF or post-MI, the treatment effects of spironolactone and eplerenone were similar, regardless of age. In the RALES trial spironolactone conferred a significant mortality reduction in patients ≥67 years compared with placebo, while eplerenone demonstrated no age-by-treatment interactions in the EMPHASIS-HF and EPHESUS trials.28–30
A meta-analysis of RCTs with MRAs that included 1,756 patients ≥75 years of age demonstrated a 26% risk reduction in CV death or HF hospitalisation with an MRA compared with placebo (HR 0.74; 95% CI [0.63–0.86]; p<0.001; heterogeneity p=0.52), without significant between-trial or age-related heterogeneity.31 Worsening renal function and hyperkalaemia were more frequent in patients taking MRAs, and worsening renal function – but not hyperkalaemia – occurred more frequently in elderly patients.
Sacubitril/valsartan
PARADIGM-HF enrolled almost 20% of patients aged ≥75 years, including 7.0% aged ≥80 years and 1.4% aged ≥85 years. A sub-analysis of this trial according to age categories (<55 years, 55–64 years, 65–74 years and ≥75 years) demonstrated consistent risk reduction in the primary endpoint of CV mortality or hospitalisation for HF (overall HR 0.80; 95% CI [0.73–0.87]; p<0.001) regardless of age, with a HR <1.0 in all age categories (p for interaction between age category and treatment=0.94). Age-by-treatment interactions were also non-significant for risk reduction in HF hospitalisation, CV and all-cause mortality. The rates of hypotension, renal impairment and hyperkalaemia increased with advancing age, irrespective of the treatment allocation. However, hypotension was more frequent, whilst renal impairment and hyperkalaemia were less frequent with sacubitril/valsartan compared with enalapril, and these findings were consistent across age categories.15
Sodium-glucose Cotransporter 2 Inhibitors
A sub-analysis of the DAPA-HF trial according to age groups (<55 years, 13.4% of participants; 55–64 years, 26.2%; 65–74 years, 36.2%; and ≥75 years, 24.2%) demonstrated similar risk reduction across the age span, with the corresponding HRs for the primary endpoint of risk reduction in CV death or hospitalisation for HF being <1.0 in all age groups (i.e. HR 0.87; 95% CI [0.60–1.28], HR 0.71; 95% CI [0.55–0.93], HR 0.76; 95% CI [0.61–0.95], and HR 0.68; 95% CI [0.53–0.88], respectively; p for interaction=0.76).16 There was no significant imbalance in tolerability or safety events between dapagliflozin and placebo, including elderly individuals. Predefined subgroup analysis of EMPEROR-Reduced with empagliflozin and SOLOIST WHF with sotagliflozin have also found no evidence of heterogeneity in treatment effects according to age.32,33
Ivabradine
A sub-analysis of the SHIFT trial stratified by age categories (<53 years, 53 years to <60 years, 60 to <69 years and ≥69 years), has shown that the relative risk of the primary endpoint (CV death or hospitalisation for worsening HF) was significantly reduced with ivabradine in all age groups (i.e. by 38% in the patients <53 years (HR 0.62; 95% CI [0.50–0.78]; p<0.001) and by 16% in patients ≥69 years (HR 0.84; 95% CI [0.71–0.99]; p=0.035).34 Up-titration of ivabradine resulted in similar reduction in heart rate in all age groups (by 11 BPM). Bradycardia, AF and phosphenes occurred at a similar rate regardless of age but were more frequently observed in patients receiving ivabradine.34
Digoxin
Available evidence indicates that digoxin improves functional status and quality of life in patients with HF and reduces total and hospitalisations for HF but has no favourable effects on mortality.35 Post-hoc analysis of the DIG trial suggested that digitalis may be less effective in the elderly HF patients and that they may experience greater risk of adverse effects because of lower lean body mass, which may cause higher concentrations of the drug in the myocardium. In addition, the adverse effects of digitalis can be worsened by renal impairment and electrolyte imbalance.36 Accordingly, keeping serum digoxin levels in a narrow range between 0.5 and 0.9 ng/dl may result in a significant 23% reduction in all-cause mortality, including patients aged ≥70 years.37 However, this requires careful titration and monitoring of serum digoxin levels, which may be challenging in clinical practice.37
Vericiguat and Omecamtiv Mecarbil
A prespecified subgroup analysis of the VICTORIA trial has suggested that vericiguat may be less effective in patients aged >75 years compared with younger individuals, but this observation may need to be further explored before reaching conclusions.38 The GALACTIC-HF trial has not suggested differences in treatment effects of omecamtiv mecarbil according to age.39
IV Iron
Elderly patients are at risk of developing anaemia because of the higher prevalence of comorbidities (e.g. renal dysfunction and malignancies), poor diet (low iron, folate, B12 intake) and concomitant use of medications that increase the risk of bleeding (aspirin, oral anticoagulants, non-steroidal anti-inflammatory drugs). Anaemia is associated with worse prognosis in HF and is responsible for reduced exercise tolerance and worsening of myocardial ischaemia.40,41 The FAIR-HF trial showed that treatment with ferric carboxymaltose in HF and iron deficiency improves New York Heart Association Class, 6-minute walk test and quality of life in patients aged ≥69.7 and <69.7 years, with no difference in adverse events and mortality between the two groups.42
Real-world Data on Drug Treatments
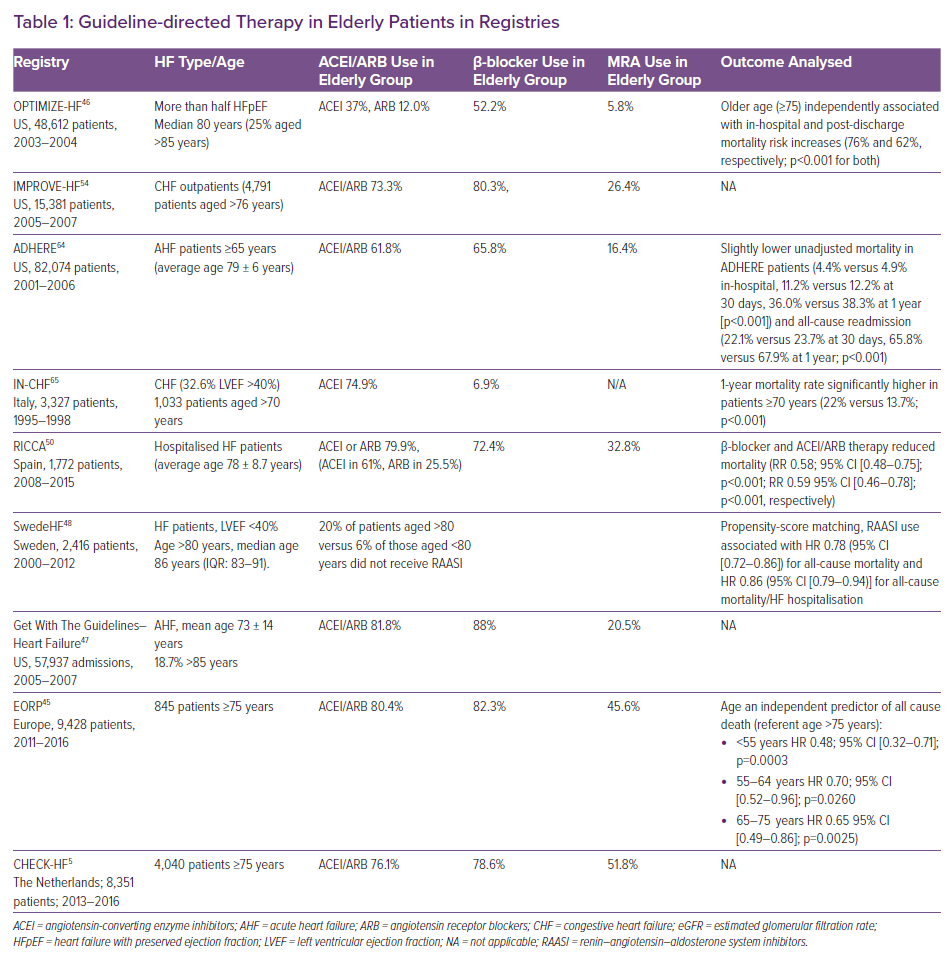
Real-world data from registries and observational studies underscore the significantly higher mortality and hospitalisation rates in older individuals with HFrEF.43,44 Indeed, the European EORP LT-HF registry has shown that all-cause mortality and all-cause hospitalisation increase with advancing age in both sexes.45 Similarly, the OPTIMIZE and GWTG registries in the US indicate that older age is independently associated with higher in-hospital and post-discharge mortality.46,47 Notably, registries confirm the findings of clinical trials that beneficial effects of GDT, including β-blockers and ACEI/ARB are not attenuated by age. In the propensity-matched analysis of the SwedeHF registry, renin–angiotensin–aldosterone inhibitor (RAASI) and β-blocker therapy was associated with a similar reduction in morbidity and mortality and no apparent association with risk of syncope-related hospitalisation in HFrEF patients aged >80 years, compared with younger individuals.48,49 Similarly, the Spanish RICCA registry has shown that β-blockers and ACEI/ARB therapy significantly reduced mortality in the elderly.50 This observation is in keeping with the results of the OPTIMIZE registry, which have shown a 23% lower mortality in elderly HFrEF patients receiving a β-blocker without evidence of an age-by-treatment interaction (p=0.87).46 The issue of potentially lower tolerability of β-blockers in the elderly was addressed in the COLA II observational study, in which over 1,000 patients aged ≥70 years were followed after initiating treatment with carvedilol. The study has shown that >80% of participants continued treatment for ≥3 months, without evidence of significant adverse effects that would require drug discontinuation.51
Attaining evidence-based target doses of HF medications in the elderly is often challenging because of the limitations discussed above. In a recent US registry, most eligible HFrEF patients did not receive target doses of medical therapy at any point during the follow-up, and few patients had doses increased over time.52 This study demonstrated that advancing age was not an obstacle to the use or up-titration of ACEI/ARBs, but older age was independently associated with a lower likelihood of initiation or dose intensification of β-blockers and angiotensin receptor–neprilysin inhibitors at 12-month follow-up.52 A recent sub-analysis of the BIOSTAT-CHF trial on the association between the achieved dose of HF medications and mortality and/or HF hospitalisation across the age spectrum demonstrated that attaining higher doses of ACEI/ARBs was associated with improved outcomes, regardless of age.53 However, achieving higher doses of β-blockers was only associated with improved outcome in those aged <70 years, but not in older patients (≥70 years).
Despite the encouraging results, registries also reveal a more concerning side of under-prescription and underuse of GDT among elderly patients for reasons that remain poorly understood (Table 1). In the EORP LT-HF registry, crude GDT utilisation rates were lower in women than in men (all differences p ≤0.001) at all ages, but age >75 years was identified as an independent predictor of GDT underuse.45 In the OPTIMIZE registry, all GDT were prescribed less frequently at discharge to eligible patients >75 years than to those <75 years.46 Similar findings were observed in the IMPROVE-HF and GWTG registries.54,47 Likewise, in the Dutch CHECK-HF registry, each 10-year increase in age was associated with a decline in the probability of receiving MRAs, β-blockers, RAASI or ivabradine, by 10%, 12%, 29% and 21%, respectively.5 At the same time, the probability of receiving diuretics increased by 32% with each decade of age. Of note, patients of older age were less likely to receive the recommended target doses of GDT medications compared with younger individuals.5
Practical Approaches to Pharmacotherapy of HFrEF in Older Patients
Natriuretic Peptide Testing
Current guidelines recommend the use of natriuretic peptide testing – B-type natriuretic peptide (BNP) and N-terminal pro-BNP (NT-proBNP) – in the diagnostic assessment of patients with HF regardless of age.55 However, interpretation of test results may be challenging in the elderly given that natriuretic peptide levels tend to increase with aging, and the presence of comorbidities, such as AF or renal dysfunction. Indeed, in patients aged >80 years with acute dyspnoea, BNP was shown to be of limited clinical utility in discriminating cardiac versus respiratory origin of dyspnoea when added to the multifactorial prediction model.56 Age-specific cut-off values have been suggested to increase the predictive value of natriuretic peptides in the elderly. A study has shown that using age-stratified NT-proBNP cut off values (i.e. 50 pg/ml in patients <50 years, 75 pg/ml in those aged 50–75 years, and 250 pg/ml in those aged >75 years) considerably improved diagnostic performance, with an excellent negative predictive value for exclusion of reduced left ventricular systolic function.57
The use of natriuretic peptides to guide or intensify GDT remains controversial as clinical trials did not demonstrate improved outcomes with this strategy.58–60 In particular, the TIME-CHF trial failed to show benefits for overall survival or HF-free survival with NT-proBNP guided medical therapy compared with standard care in individuals ≥75 years of age.61
Guideline-directed Medical Therapy
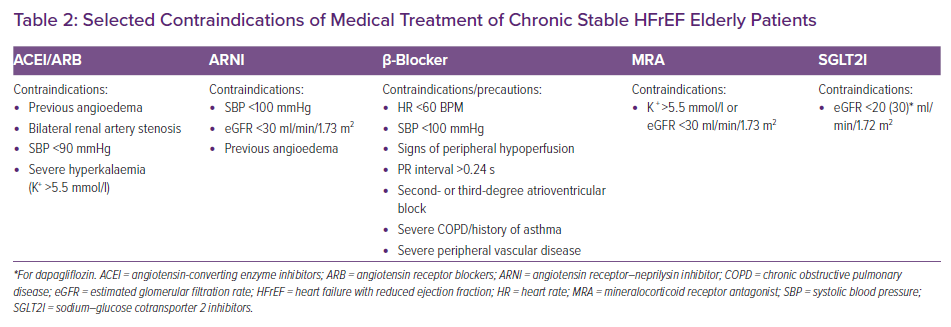
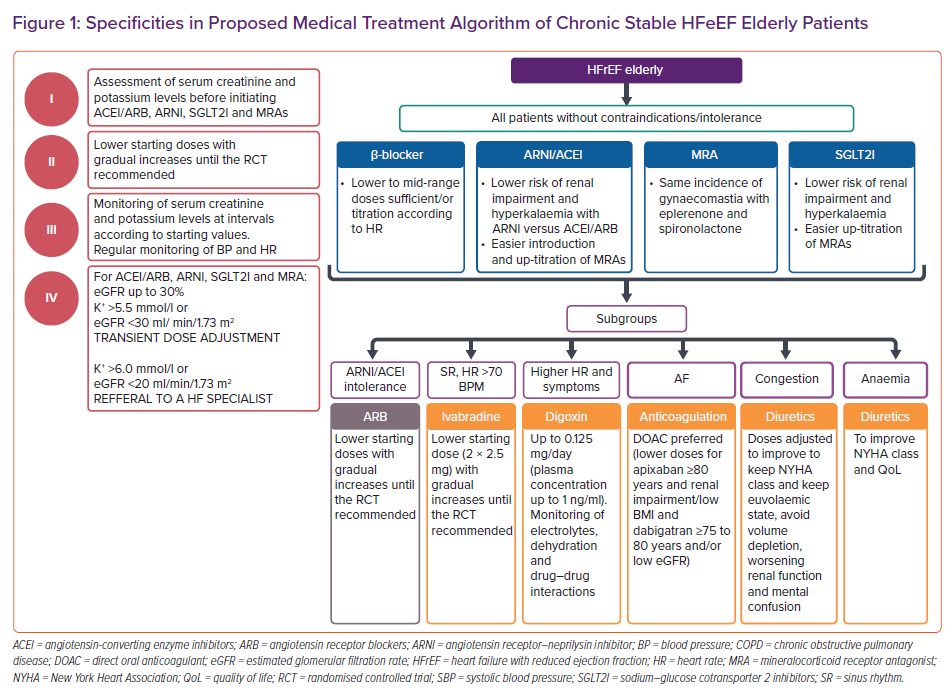
Despite the proven benefits of medical therapies for HFrEF (except, perhaps, vericiguat) and reassuring safety profile of most drugs, there remains a reluctance in the real-world clinical practice to prescribe and up-titrate these medications in older people. This may be the result of (mis)understanding that elderly individuals have lower tolerance and greater propensity for developing adverse drug reactions, in particular in the presence of comorbidities that interfere with drug metabolism. It may also reflect issues around access to specialised care, difficulties in the management of multiple medications, patient preferences, and other non-medical considerations. In order to overcome these issues, it is prudent to commence HF medications in older patients at lower doses then to slowly and carefully up-titrate to target doses to prevent intolerance and adverse drug reactions.62 (Figure 1 and Table 2).
Assessment of serum creatinine and potassium levels is recommended before initiating ACEI/ARB and MRAs and monitoring is needed at intervals set according to baseline renal function and potassium concentration. Monitoring should be intensified in face of changes in clinical status that may increase the risk of worsening renal function and hyperkalaemia. An acute decline in estimated glomerular filtration rate is frequent following initiation of ACEI/ARB and should not be the reason to discontinue treatment, but transient dose adjustment may be required. Given that sacubitril/valsartan carries a lower risk of renal impairment and hyperkalaemia it may be the preferred drug choice over ACEI/ARB. This may also allow for easier introduction and up-titration of MRAs. Digoxin should only be considered in select patients for symptom relief and prevention of repeat HF hospitalisations, but only if careful up-titration and monitoring of serum drug levels can be performed. Diuretic doses also need to be adjusted to keep a euvolemic state whilst avoiding volume depletion, worsening renal function and mental confusion.
Since polypharmacy is frequent among the elderly, simplification of the treatment scheme is highly recommended. It is advisable to review prescribed medications and discontinue drugs that may precipitate worsening HF symptoms (e.g. thiazolidinediones, Class I antiarrhythmic medications, dronedarone, calcium channel blockers expect amlodipine and felodipine, etc.) and substitute them with safer choices. Patients also need to be warned about caveats of over-the-counter drugs (e.g. non-steroidal anti-inflammatory drugs) and herbal remedies that may aggravate HF symptoms and cause severe drug interactions. Cognitive impairment and HF frequently coexist and a multidisciplinary team approach is recommended. The use of adherence aids and greater involvement of family members and caregivers could improve self-care and adherence to HF treatment.63
Call for Action
With the aging global population and the growing burden of HF, future research should focus on providing more granular analyses on how to best approach medical and device therapies in elderly patients. These should take into account biological differences, difficulties in care delivery and issues relevant to patients’ values and perspectives. Over the past decades, the number of old and very old patients enrolled in RCTs has increased, but their broader representation should be encouraged to obtain better insights into the efficacy and safety of investigated treatments. In addition, more information is needed from real-world practice on reasons for underuse of the available treatment options in older populations. Improved education of healthcare professionals, wider provision of specialised centres for multidisciplinary HF care and stronger implementation of GDT in vulnerable patient groups, may prove to be the way to ‘add years to life – and life to years’ in elderly patients with HF.42