Heart failure (HF) has an estimated global prevalence of 1–2% and is a major cause of morbidity and mortality.1 Despite a growing armoury of evidence-based therapies, it is estimated that 5–10% of patients deteriorate into advanced HF, which has a poor prognosis if not treated with advanced therapies.2
Abnormal central haemodynamics are a hallmark of advanced HF and right heart catheterisation (RHC) is an important procedure for identifying advanced disease and guide the timing of advanced therapies, such as heart transplantation or left ventricular assist device (LVAD) implantation.3,4
A range of measured and derived haemodynamic variables is used to determine the degree of haemodynamic impairment. Recently, the aortic pulsatility index (API) – a haemodynamic variable derived from systemic pulse pressure divided by pulmonary capillary wedge pressure (PCWP) – has been proposed as a new marker of left heart performance with evidence of prognostic predictive abilities superior to the established haemodynamic measurements in patients with acute decompensated HF.5
API was introduced only recently and the association between API and long-term prognosis in advanced HF has not been described. The primary aim of this study was to test if the index had long-term prognostic predictive value in advanced stable HF, secondary to test the association between API and central haemodynamic measurements.
Methods
Patients and Study Design
A cohort of HF patients referred for right heart catheterisation (RHC) at the Department of Cardiology at Copenhagen University Hospital, Rigshospitalet from 1 January 2002 to 31 October 2020 was investigated. Patients were referred for evaluation for advanced therapy, i.e. implantation of an LVAD, total artificial heart implantation or heart transplantation, or as a part of assessment of advanced HF. All RHCs were performed in the catheterisation laboratory as non-urgent procedures.
Patients were identified through the hospital’s cardiac catheterisation database, from which data were extracted and linked to the patients’ medical records and the hospital’s echocardiography database. Patients were included if they had a documented left ventricular ejection fraction (LVEF) <45%. Patients were not required to have symptoms of advanced HF at the time of referral. However, these patients were included as they had recently experienced advanced HF symptoms and/or their HF condition was considered severe to the extent that referral for evaluation for advanced therapies was justified.
All patients were considered to have an element of chronic HF, but some patients could be in the state of worsening HF with decompensation during the time of their haemodynamic evaluation. Unstable patients requiring intensive care, ventilation or temporary mechanical support were excluded from the analysis. In cases of repeated RHC during the time period, only data from the first catheterisation was used.
Patients treated with mechanical circulatory support or heart transplantation at the time of catheterisation were excluded from the study as were patients aged under 16 years and those with congenital heart defects. Patients were required to be on optimal medical therapy (as tolerated) before referral.
The study complies with the Declaration of Helsinki. The research protocol was approved by the local research ethics committee (3-3013-1365/1) and the data protection agency (P-2020-1087). Individual patient consent was not required because of the retrospective nature of the study.
Haemodynamic Evaluation
All RHCs were performed in the cardiac catheterisation laboratory by four experienced physicians. A Swan-Ganz catheter was used with zeroing and calibration of the pressure transducer performed before measurements. The catheter was inserted in the internal jugular or the femoral vein, and correct placement was evaluated by fluoroscopy and by visualisation of pressure curves on a monitor.
The following haemodynamic variables were collected: PCWP; mean pulmonary artery pressure (MPAP); central venous pressure (CVP); cardiac output (CO) using the thermodilution technique; systolic blood pressure (SBP); diastolic blood pressure (DBP); and heart rate. Blood pressure was measured noninvasively using a semi-automated cuff method.
Derived variables were calculated as follows: API was determined using the formula:

Cardiac index was determined as CO divided by the body surface area. Body surface area was determined using the DuBois method.
Mean arterial pressure (MAP) was estimated using the formula:

Stroke volume index (SVi) was calculated using the formula:

Pulmonary artery pulsatility index (PAPi) was determined using the formula:

Statistical Analysis
API and N-terminal proB-type natriuretic peptide (NT-proBNP) were non-normally distributed and were therefore log-transformed for analysis. API and NT-proBNP were reported as medians with interquartile range (IQR). We reported all other continuous variables as mean ± standard deviation (SD) and categorical variables as numbers and/or percentages. Univariable and multivariable linear regression models were constructed including relevant haemodynamic measurements and important clinical variables. Variables that presented with a p value of <0.05 in univariable regression models were included in the multivariable regression model.
The follow-up date was set at 31 October 2020. Events were defined as: death (all cause); implantation of a durable LVAD; total artificial heart implantation; or undergoing heart transplantation. Implantation of an LVAD was carried out as a bridge to transplantation or as destination therapy.
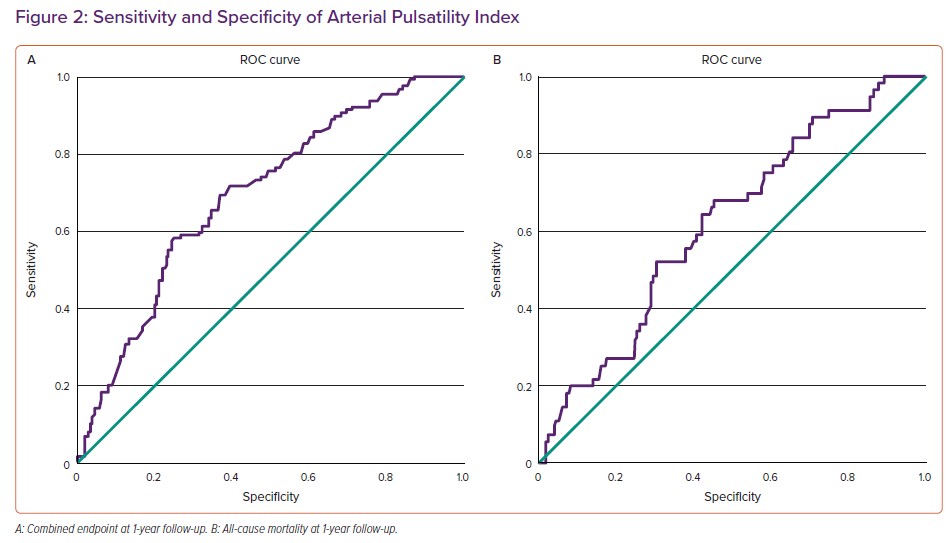
Cox proportional hazards models were used to determine API’s ability to predict: the combined endpoint of all-cause mortality, implantation of a durable LVAD, total artificial heart implantation or heart transplantation; or all-cause mortality while censoring patient data at the time of implantation of LVAD, total artificial heart implantation or heart transplantation. Patients were divided into tertiles according to API for the construction of Kaplan-Meier curves. Receiver operator characteristic (ROC) curves for 6 months (Supplementary Material Figures 1A and 1B) and 1-year follow-up time were constructed and area under the curve (AUC) was determined.
Two-sided p-values were used, and p<0.05 was considered statistically significant. Statistical analyses were performed using SPSS (version 25, IBM).
Results
Clinical Characteristics
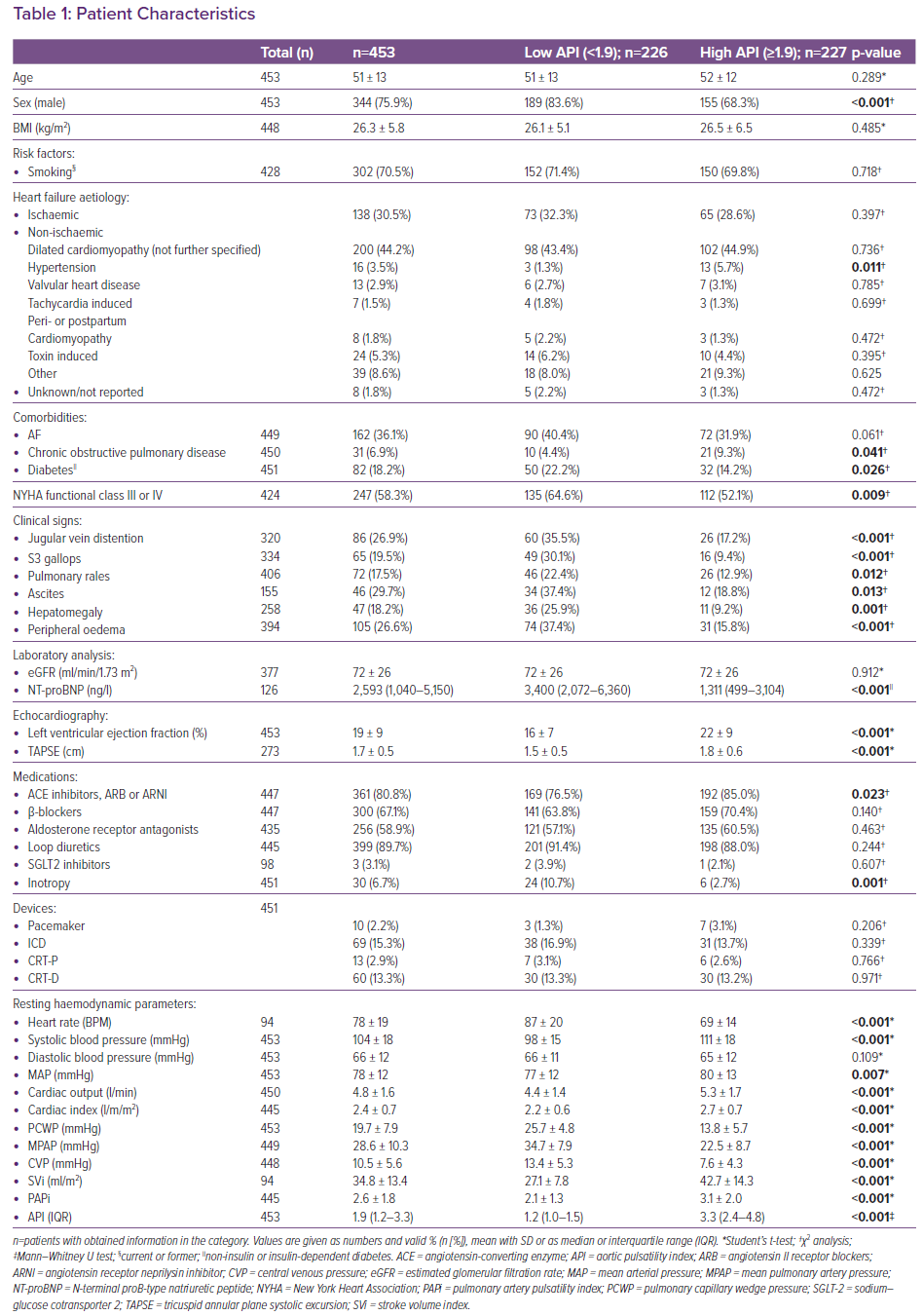
API was calculated for 453 patients with advanced HF undergoing haemodynamic evaluation. Clinical features are summarised in Table 1. Median API was 1.9 (1.2–3.3) and mean follow-up was 9.1 years. The total cohort was characterised by low LVEF (19% ± 9%), a majority of men (76%), a relatively young age (51 ± 13 years) and an above average BMI of 26 ± 6 kg/m². Thirty-one per cent had ischaemic HF aetiology. Only nine patients had severe mitral regurgitation (2%) and four had moderate-to-severe mitral regurgitation (1%).
Patients were divided into two groups according to low (<1.9) or high (≥1.9) API, where 226 patients had low API and 227 had high API. Comparing the two groups, we found that patients with low API were more often men (84% compared to 68% in the group with high API) and in a higher NYHA class (with 65% of patients with low API classified as NYHA III/IV versus 52% of patients with high API; p=0.009).
Patients with low API showed clinical signs of congestion more frequently that patients with high API, with 63% of patients with low API having at least one clinical sign of congestion compared to 38% of patients with high API. Patients with low API had higher median NT-proBNP levels (3,400 versus 1,311 ng/l; p<0.001) and lower LVEF (16% versus 22%; p<0.001).
Tricuspid annular plane systolic excursion was significantly lower in patients with low API than in those with high API (1.5 versus 1.8; p<0.001). The eGFR was generally mildly decreased with no differences in renal function between groups.
There were no significant differences between groups in standard of care HF medication except that patients with low API were less often prescribed angiotensin-converting enzyme-inhibitors, angiotensin II receptor blockers or angiotensin receptor neprilysin inhibitors (ARNIs) than patients with high API (77 versus 85%; p=0.023). There were no differences between groups regarding prescriptions of ARNI (six patients in each group).
Patients with low API received inotropic support more often (defined as patients receiving infusion of inotropic medications – dobutamine, dopamine, milrinone or norepinephrine – at the time of the haemodynamic evaluation) than patients with high API (11 versus 3%; p=0.001). Patients with low API were significantly more haemodynamically deranged for almost all measured parameters.
Compared with patients with high API, patients with low API had a higher heart rate (87 ± 20 BPM versus 69 ± 14 BPM; p<0.001), lower SBP (98 ± 15 mmHg versus 111 ± 18 mmHg; p<0.001) and lower MAP (77 ± 12 mmHg versus 80 ± 13 mmHg; p=0.007). Their CO (4.4 ± 1.4 l/min versus 5.3 ± 1.7 l/min; p<0.001), cardiac index (2.2 ± 0.6 l/min/m2 versus 2.7 ± 0.7 l/min/m2; p<0.001) and SVi (27.1 ± 7.8 versus 42.7 ± 14.3; p<0.001) were reduced. PCWP (25.7 ± 4.8 mmHg versus 13.8 ± 5.7 mmHg; p<0.001), MPAP (34.7 ± 7.9 mmHg versus 22.5 ± 8.7 mmHg; p<0.001) and CVP (13.4 ± 5.3 mmHg versus 7.6 ± 4.3 mmHg; p<0.001) were elevated. Patients with low API had significantly lower PAPi (2.1 ± 1.3 versus 3.1 ± 2.0; p<0.001) than those with high API.
Supplementary Material Table 1 shows patient characteristics stratified according to NYHA class.
Association Between API and Haemodynamic and Clinical Variables
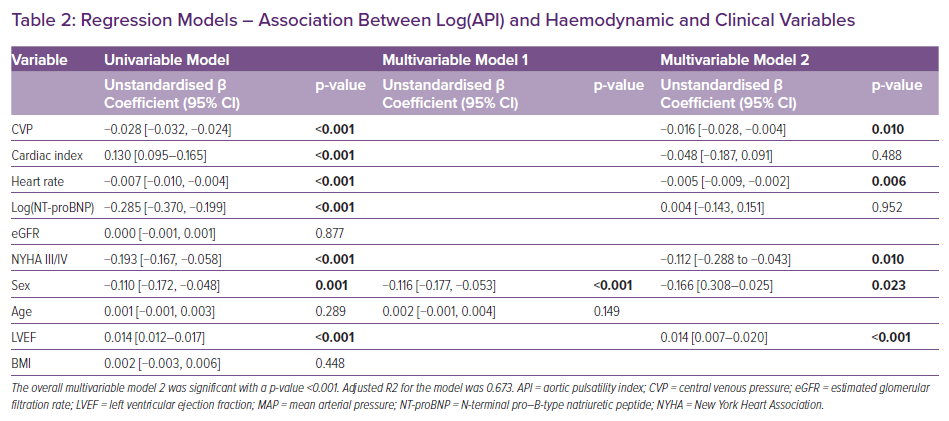
Log(API) was significantly associated with CVP (p<0.001), cardiac index (p<0.001), heart rate (p<0.001), log(NT-proBNP) (p<0.001), NYHA III/IV (p<0.001), male sex (p=0.001) and LVEF (p<0.001) in univariable linear regression analysis. Age, eGFR and BMI were not associated with log(API). In an intermediate multivariable model including only age and sex, male sex was strongly associated with log(API) (p<0.001).
In multivariable analysis including all significant variables from the univariable analysis, CVP (p=0.01), heart rate (p=0.006), NYHA III/IV (p=0.010), male sex (p=0.023) and LVEF (p<0.001) were found to be significantly associated with log(API). Cardiac index and log(NT - proBNP) lost their statistical significance in the multivariable model (Table 2).
API and Outcome
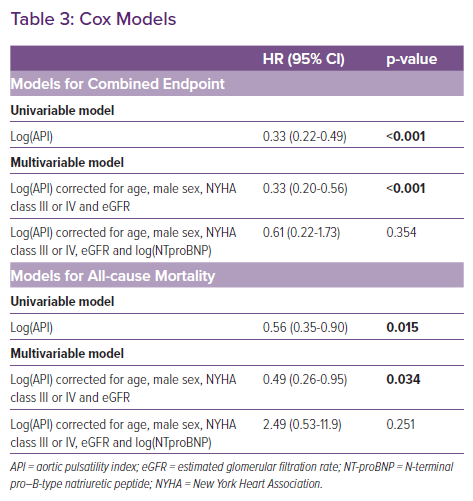
Log(API) predicted freedom from the combined endpoint of death, LVAD implantation, total artificial heart implantation or heart transplantation (HR 0.33; 95% CI [0.22–0.49]; p<0.001) and freedom from all-cause mortality (HR 0.56; 95% CI [0.35–0.90]; p=0.015; Table 3).
When adjusting for age, sex, NYHA class and eGFR in multivariable Cox analysis, log(API) remained independently associated with freedom from the combined endpoint (HR 0.33; 95% CI [0.20–0.56]; p<0.001) and all-cause mortality (HR 0.49; 95% CI [0.26–0.96]; p=0.034). However, the associations did not reach statistical significance when further adjusting for log(NT-proBNP) for either the combined endpoint (HR 0.61; CI [0.22–1.73]; p=0.354) or all-cause mortality (HR 2.49; CI [0.53–11.8; p=0.251). It had no significant effect on the results of any of the Cox models if data from patients on inotropy were excluded from the analysis.
There was no statistically significant interaction effect of NYHA class (NYHA I–II versus NYHA III–IV) on log(API)’s ability to predict freedom from the combined endpoint (p=0.789) and all-cause mortality (p=0.805) in univariable Cox models. There was a statistically significant interaction effect of aetiology (ischaemic versus non-ischaemic) on the ability of log(API) ability to predict freedom from the combined endpoint (p=0.030) but not all-cause mortality (p=0.185). When patients were divided according to aetiology in two separate Cox models, log(API) remained a significant predictor of freedom from the combined endpoint for both ischaemic (HR 0.09; CI [0.03–0.26; p<0.001) and non-ischaemic (HR 0.51; CI [0.27–0.96; p=0.036) patients (Supplementary Material Table 2).
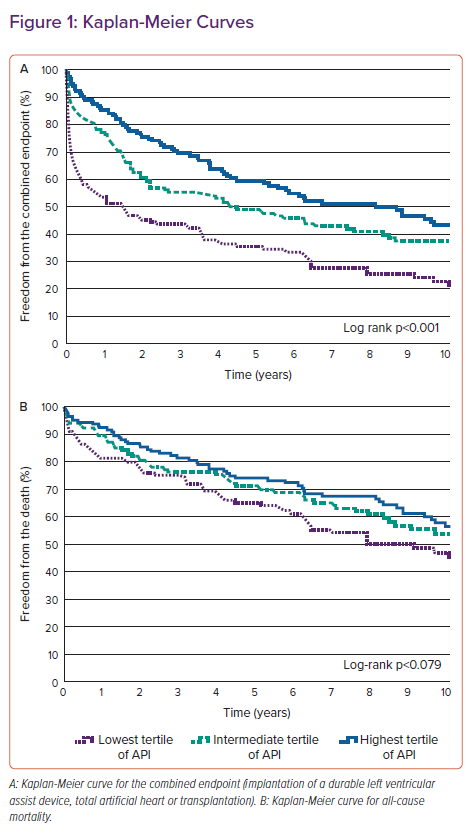
A Kaplan-Meier curve demonstrated there were significant differences (log rank p-value <0.001) between patients grouped in tertiles according to API for the combined endpoint (Figure 1A). Median time to the combined endpoint was 1.5 years for the lowest tertile and 8.1 years for the highest. There were no statistically significant differences between subgroups for all-cause mortality (log rank p=0.079; Figure 1B).
ROC curves showed an AUC of 0.694 (95% CI [0.642–0.747]; p<0.001) for the combined endpoint at 1-year follow-up and an AUC of 0.617 (95% CI [0.543–0.692; p=0.005) for all-cause mortality at 1-year follow-up (Figure 2). Furthermore, additional ROC curves for the combined endpoint and all-cause mortality at 6 months’ follow-up showed an AUC of 0.706 (95% CI [0.651–0.760]; p<0.001) for the combined endpoint and an AUC of 0.618 (95% CI [0.553–0.703]; p=0.012) for all-cause mortality (Supplementary Material Figures 1A and 1B).
Discussion
This study aimed to evaluate the novel haemodynamic variable API and its ability to predict long-term prognosis in advanced HF. To our knowledge, it is the largest study to analyse the prognostic impact of API and the only study with long-term follow-up.
We demonstrated that log(API) was strongly associated with right-sided filling pressures in univariable and multivariable models. Furthermore, we demonstrated that log(API) was a significant predictor of freedom from the combined endpoint and all-cause mortality in univariable Cox models and an independent factor when adjusting for multiple known variables associated with a worse outcome in HF.
Pulmonary artery pulsatility index (PAPi), a derived haemodynamic parameter calculated as pulmonary artery pulse pressure divided by central venous pressure, has recently been established in clinical settings as a marker of right ventricular function with prognostic implications.6 API could be understood as a marker in line with PAPi but linked to the systemic rather than the pulmonary circulation. API is derived from PCWP (i.e. an estimate of preload of the left ventricle [LV]) and pulse pressure, which is a function of stroke volume and LV afterload, and thus API could be an integrated measure for LV function taking into consideration the loading conditions. In this study, we found an increased event rate as API decreased. This is in line with published literature, where low pulse pressure and high left ventricular filling pressures are known individual predictors of a worse prognosis in advanced HF. 7,8
There is a growing list of derived haemodynamic variables with prognostic implications. One such is cardiac power output (CPO). CPO is calculated as mean arterial pressure multiplied by CO and divided by 451; it integrates flow and pressure and could be viewed as the hydraulic pumping ability of the heart. It has been shown that resting CPO serves as a powerful prognostic factor in a broad spectrum of patients with acute cardiac disease as well as in ambulatory patients with advanced HF.9,10 In contrast to CPO, API does not take CO directly into account. However, since there is greater uncertainty on CO measurements, this could be an advantage of API, possibly making it a more accurate estimate. Future studies should explore this further.
A recent study by Belkin et al. examined data from the ESCAPE trial and showed that API was a better predictor of clinical outcome than traditional haemodynamic variables at 6 months’ follow-up in a cohort of 189 patients with acute decompensated HF.5 In another study, Belkin et al. examined a cohort of 244 patients with acute, chronic and worsening HF who were all treated with milrinone and found that low API was strongly associated with needs for advanced therapies or with death at 30-day follow-up.11
Our study demonstrated that API was an independent predictor of clinical outcomes in multivariable Cox proportional hazards models, though not when adjusting for log(NT-proBNP), which is also known to be a strong prognostic predictive factor in advanced HF.12 We speculate that this could be owing to lack of power, since NT-proBNP was reported for only one in four patients, leaving the sample size for analysis moderate. Further studies are required to determine if API provides prognostic information beyond that of NT-proBNP.
In contrast to Belkin et al., we only found acceptable AUC values for the combined endpoint and additionally poor discrimination abilities of API in ROC curves for all-cause mortality. It is important to note that where Belkin’s studies investigated mostly acute, decompensated HF patients, our cohort was mainly elective, stable patients. Furthermore, we investigated long-term prognosis (years) compared to much shorter (days to months) in Belkin’s two studies, and this could be a possible explanation for why our results differ. This is supported by the fact that when constructing ROC curves for 6 months follow-up time (as used by Belkin et al. analysing the ESCAPE trial), our AUC for the combined outcome (0.706) was similar to Belkin’s (0.708).
In the current study, low API was associated with generally sicker patients, as they had more frequent signs of congestion, more deranged haemodynamics and a greater need for inotropy. Our findings suggest that calculation of API could be useful for risk stratification in patients with advanced HF.
Calculation of API in patients undergoing RHC is simple and may add to the characterisation of the LV and, in turn, provide prognostic information in patients with advanced HF. API has recently been introduced and the impact of API in HF should be confirmed in other studies, thresholds clearly defined and added value determined before clinical recommendations for use can be made.
Limitations
Several limitations in this study must be acknowledged. Because of its retrospective nature, conclusions on cause-effect relationships cannot be made. The study included a selected HF patient population with an indication for RHC referred for evaluation at a single specialised centre, which introduces the risk of selection bias. The population investigated were much younger than HF patients on average, which may limit the ability to extrapolate our results to the general HF population.
Sodium-glucose cotransporter 2 (SGLT-2) inhibitors were recommended for HF patients with reduced ejection fraction in 2021 European Society of Cardiology guidelines for the diagnosis and treatment of acute and chronic heart failure.13 Since SGLT-2 inhibitors may reduce PCWP and only three patients in our cohort were taking SGLT-2 inhibitors, our data may not represent patients on updated guideline-recommended HF medication.14 Future studies should include patients treated with this drug class.
Systolic and diastolic blood pressure were measured noninvasively by semiautomatic blood pressure cuff and we cannot exclude the possibility off less consistent measurements than those that would have been obtained from an intra-arterial catheter.
Conclusion
The novel haemodynamic measurement API predicted freedom from the combined endpoint and all-cause mortality even when correcting for known variables associated with a worse outcome in HF. Further studies should explore the prognostic value of API in advanced, stable HF.
Click here to view Supplementary Material.
Clinical Perspective
- Calculation of the aortic pulsatility index is simple and may add to the characterisation of the left ventricle.
- The aortic pulsatility index is independently associated with freedom from advanced therapies or death in long-term follow-up.
- The aortic pulsatility index could be useful in risk stratification in patients with advanced heart failure.