Heart failure (HF) is estimated to affect more than 64 million people worldwide, and its prevalence continues to grow.1 Among the reasons for the increasing prevalence of HF, the ageing of the population is probably one of the most important and is one explanation for the persistently poor prognosis and increasing burden of HF-related hospitalisations.2–8
Broadly speaking, the results of randomised control trials (RCTs) are poorly generalisable to daily clinical practice, limiting the implementation of their findings.9 Older patients are not explicitly excluded from RCTs, but the median age of patients included in such studies is systematically below 70 years and thus poorly representative of the general HF population.10
According to the current European HF guidelines age is not a contraindication to guideline-directed medical therapy (GDMT), and data do not demonstrate a lack of benefit of evidence-based medications in older adults.11–16 Nevertheless, under-implementation of treatment in older individuals is extensively encountered in the literature, which could be explained by routine clinical considerations, such as perceived contraindications or low tolerability, the risk of drug–drug interactions in polytherapy, patients’ preferences and clinical inertia.17–22 The fear of side-effects or the perceived lack of benefit derived from a focus on symptoms rather than prognosis also limits treatment implementation in older patients.23 Moreover, weaker evidence supporting the incremental prognostic effect of dose optimisation in older patients may lead to a reluctance on the part of clinicians to consider dose titration.24–26 In this review, we provide an overview of the treatment of HF with reduced ejection fraction (HFrEF) in older patients and summarise the evidence regarding the efficacy of these HF treatments.
Real-world and Randomised Clinical Trials: Two Distinct Entities
Phenotypic classification of HF remains anchored to the categorisation of ejection fraction (EF).11 Among the three categories of HF, only HFrEF has established treatments based on solid evidence derived from multiple RCTs. However, poor generalisability is one of the major limitations to the applicability of RCT results in real-world practice, with age being a typical example. The mean age of HF patients in most developed countries is >70 years and the prevalence of HF increases with age, ranging from 2% in the general population to >10% among people aged >70 years.6,19 Moreover, a considerable portion of the general HF population is aged ≥80 years; for example, up to 30% of the SwedeHF HFrEF population was aged >80 years and 15% of patients enrolled in the GTWG-HF were aged >85 years.27,28 However, the scenario in RCTs is completely different. In one large meta-analysis of the results from major RCTs on β-blockers, the median age was 64 years.14 In the only study designed to assess the efficacy of β-blockers in older HF patients, namely the SENIORS trial, one inclusion criterion was age ≥70 years, and the mean subject age was 76 years.24 Similarly, in former RCTs on renin–angiotensin system (RAS) inhibitors and mineralocorticoid receptor antagonists (MRA), the mean age was well below 70 years.26,29–33 The progressive aging of the general HF population should have been translated into a change in the patients eligible for inclusion in RCTs. Instead, in the most recent studies, the mean age at enrolment ranged from 63 years in the PARADIGM-HF trial to 67 years in the VICTORIA study.34–37
When not specified by exclusion criteria, the low rate of inclusion of older populations in RCTs could be explained by a reduced rate of referral of older individuals for cardiology specialist care and the frequent coexisting conditions that may preclude or discourage the inclusion of these individuals in RCTs (i.e. cardiovascular and non-cardiovascular comorbidities, frailty issues, polypharmacy).19,20,38 Whatever the cause, the widening discrepancy between RCTs and the real world opens up the debate of the generalisability of trial results in the routine management of HF, particularly in older patients.
Adherence to Guideline-directed Medical Therapy in Older Patients: Data from Registries
Age is a recognised major determinant of low adherence to GDMT in HFrEF.17,18,20,21 In the CHAMP-HF registry, older age was associated with the lower use of β-blockers, MRAs and angiotensin receptor–neprilysin inhibitor (ARNI), and, at the 12-month follow-up, dose maximisation was less likely with increasing age.18,39 Similarly, in the US GWTG-HF registry, there was a decreasing gradient in the use of GDMT with increasing age, although the authors correctly highlighted that the prescription rate was high overall also in the oldest category (i.e. 79% and 83% of patients >85 years old were on angiotensin-converting enzyme inhibitors [ACEi] and β-blockers, respectively).28
There is a similar apparent reticence in Europe to implement treatments in older patients. For example, in 2009, octogenarians in the Euro Heart Failure Survey II were less likely to be treated compared with younger age classes, with only 76% of those aged >80 years treated with a RAS inhibitor, 53% treated with β-blockers and 38% treated with an MRA.19 Octogenarians had a heavier burden of comorbidities, including anaemia, chronic obstructive pulmonary disease and chronic kidney disease, more frequent indicators of frailty and less favourable socio-demographic conditions.19 All these aspects should be considered as partial explanations for the lower adherence to GDMT among patients in older age categories. However, recent data collected in the CHECK-HF registry attested to an overuse of diuretics in older patients, with under-prescription of evidence-based drugs for the treatment of HFrEF.17 The inverse association between age and the use of medication was confirmed for ACEi, β-blockers and MRA even after extensive adjustment, supporting that, beyond the obvious higher prevalence of comorbidities or socio-demographic factors limiting treatment implementation, age per se limits the application of GDMT in HFrEF.17
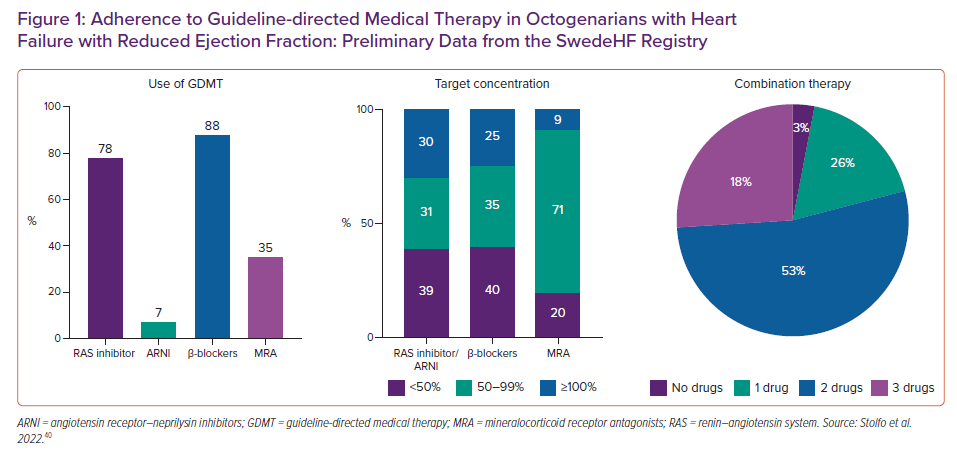
Recent data from the SwedeHF Registry provided a comprehensive overview of the current treatment approach in older (i.e. ≥80 years) patients with HFrEF.40 Of 27,430 patients with HFrEF, 35% were aged ≥80 years. The use of treatments decreased progressively with increasing age: for example, the use of RAS inhibitor/ARNI, β-blockers and MRA, was 95%, 95% and 54%, respectively, for those aged <70 years, compared with 80%, 88% and 35%, respectively, for those aged ≥80 years (Figure 1). Devices were similarly underused in older patients, and older patients were less likely to be treated with target doses of GDMT or to receive multiple drugs in combination (only 26% among those aged ≥80 years).40
There are several reasons that may explain the lower use of treatments among older patients. With aging, the increasing burden of comorbidities may hamper the implementation of treatments. Chronic kidney disease, for example, may be perceived as a potential contraindication for treatment with an RAS inhibitor or ARNI. However, in a previous analysis from the SwedeHF Registry, 66% of HFrEF patients with severe impairment of renal function were treated with RAS inhibitors, suggesting that trial criteria for a low estimated glomerular filtration rate are not a strong deterrent for the use of RAS inhibitors.41 Potential reasons for β-blocker underuse in the older population may be related to safety concerns, in particular the risk of hypotensive or bradyarrhythmic events. However, in a former study from the SwedeHF Registry, no increased risk of hospitalisation for syncope, which may be a consequence of hypotension or bradyarrhythmia, was observed in older subjects.27 Moreover, dedicated studies have shown good tolerability of β-blockers in older people with HF and, in a meta-analysis of 11 HFrEF RCTs, older age was not associated with treatment discontinuation, although the median age in the RCTs was lower compared with the real-world HFrEF population.14,42,43 Other reasons for the underuse and underdosing of HF treatments in older patients may include lower socio-economic status, lower education levels and fewer referrals to specialty care. Finally, polypharmacy, which is typical of older individuals with multimorbid conditions, is another deterrent to treatment use and dose maximisation, with potential negative effects on outcome.44
Effect of Evidence-based Therapy in Older Patients with HFrEF
Although guidelines do not recommend age-related differences in medical approaches, the evidence supporting the efficacy of GDMT in older patients is weak.11 Most of the landmark RCTs generating the evidence forming the basis of the contemporary medical approach to HF, including the most recent RCTs on ARNI and sodium–glucose cotransporter 2 (SGLT2) inhibitors, enrolled younger patients, and there are very few examples of studies specifically designed for older age categories. In a meta-analysis of four RCTs enrolling patients with left ventricular dysfunction, ACEi did not affect survival or the composite risk of death/MI/HF hospitalisation in patients >75 years.13 However, only 1,066 patients were aged >75 years, compared with 11,674 aged ≤75 years, and there was no significant interaction between age and the effect of ACEi.13 Similarly, in post hoc analyses of RCTs, including the most novel classes of ARNI and SGLT2 inhibitors, age did not impact on the treatment effect.12,14,15,45,46 Post hoc analysis data from the DAPA-HF trial reported consistent (i.e. no significant interaction) effects across age categories for all the study outcomes; interestingly, the magnitude of the effect of dapagliflozin in reducing the composite endpoint of death/HF hospitalisation and the secondary endpoint of urgent HF visit/HF hospitalisation was numerically higher in those aged >75 years compared with younger age categories.45
The only study designed to assess the efficacy of β-blockers in older HF patients was the SENIORS trial (inclusion criteria age ≥70 years; mean age 76 years), which showed a significant reduction in the combined risk of death or cardiovascular rehospitalisation, but no significant effect on survival, in patients receiving β-blockers versus the placebo arm.24 Of note, most of that study cohort was aged <80 years, and approximately one-third had a left ventricular EF >35%. It is contentious whether age per se can explain the different effects of nebivolol on mortality observed in SENIORS compared with the largest benefit of β-blockers observed in other RCTs.
A recent large meta-analysis of RCTs including patients with HFrEF and sinus rhythm showed a significant benefit of β-blocker therapy in terms of all-cause mortality that was consistent across all age groups, but age attenuated the effect of β-blockers on the risk of HF hospitalisation (p for interaction<0.05).14 Similar results were observed for HF hospitalisation, albeit with a smaller effect of β-blockers in older patients.14 In older patients, the benefit of treatments in terms of improvements in symptoms and quality of life can be apparently reduced, being frequently affected by concomitant comorbid conditions and limited mobility. However, data from the DAPA-HF study demonstrated similar changes in the total symptom score on the Kansas City Cardiomyopathy Questionnaire (KCCQ-TSS) in older (i.e. ≥75 years) and younger patients (i.e. <55 years).45
The existing knowledge gap between selected cohorts included in RCTs and the real world can be filled, at least in part, by observational studies that have reproduced similar prognostic effects of GDMT for both RAS inhibitors and β-blockers in patients in older age categories.27,47
The complexity of older individuals with HFrEF can lead to a more cautious approach to the dose titration of GDMT. Moreover, the additional benefit of increasing dosing is less well-established in patients in older age categories. In the two largest RCTs comparing low (50 mg daily of losartan, 2.5–5.0 mg daily of lisinopril) versus high dose (150 mg daily of losartan, 32.5–35 mg daily of linisopril) of ACEi/angiotensin receptor blockers (ARB), patients assigned to higher doses had significantly improved outcomes than those being treated with lower doses, with no effect of age, with older (>65 years) patients having similar outcomes to younger patients.25,26 However, in clinical trials of older HFrEF patients, there is some evidence suggesting that there may not be incremental benefit from achieving target doses of β-blockers compared with lower doses. For example, in the SENIORS trial, patients on 50% of the target dose had similar outcomes to those on 100% of the target dose.24 Such observations were confirmed by the multicentre European cohort BioStat-CHF study, which demonstrated additional benefit for higher doses of RAS inhibitors in both older (≥70 years) and younger (<70 years) groups, whereas the improvements in outcome obtained with higher doses of β-blockers were limited to the younger group.20
The existence of multiple comorbidities that enhance the risk of adverse reactions, the perception of low tolerance and the concomitant polytherapy for extracardiac conditions may limit the sequential combination of evidence-based treatments in older patients. In the SwedeHF Registry, less than 20% of octogenarians were on a triple-drugs combination (Figure 1).40
No specific studies have assessed the incremental prognostic benefit of comprehensive evidence-based HFrEF therapy in older groups. However, in an indirect comparison of three of the major RCTs, the estimated gain in HF hospitalisation-free survival provided by comprehensive disease-modifying pharmacological therapy (ARNI, β-blocker, MRA and SGLT2 inhibitor) compared with conventional therapy (RAS inhibitor and β-blocker) in a hypothetical 80-year-old patient was 2.7 years, although this was less than the gain in younger patients.48
Unmet Needs and Future Directions
Older patients are rapidly becoming prevalent in the overall HF population. Older patients are associated with enormous complexity that is determined by several factors, including a greater burden of cardiac and non-cardiac comorbidities, frailty, a lower tolerance to medications and a higher risk of drug–drug interactions because of polypharmacy. All these aspects can lead to lower adherence to GDMT, even though these patients are at higher risk of poor cardiovascular and non-cardiovascular outcomes, further contributing to increasing pressures on healthcare systems, with considerable effects on financial costs.
There is a persistent mismatch between the characteristics of populations enrolled in RCTs and those of patients seen in regular daily practice. In particular, older patients have been classically excluded or largely under-represented in RCTs, questioning the evidence that supports the adoption of GDMT for HFrEF and the achievement of target doses of HF medications in the older population. Guidelines recommend a standard approach to the treatment of HFrEF, regardless of age.11 However, in current practice, as confirmed by large international HF registries, older age is a strong deterrent to the initiation and titration of treatment in HFrEF. Stronger efforts are needed to improve strategies for treatment implementation in older patients. Enrichment strategies for the inclusion of older patients in RCTs and studies specifically designed for older patient age categories could provide solid evidence on the benefit of HF treatments in this group. Moreover, the incorporation of measures of quality of life or frailty, such as the Rockwood Clinical Frailty Scale, could be helpful in estimating treatment benefit and the risk of poor tolerance/limited adherence in older patients.49
Real-world practice may benefit from a broad range of interventions encompassing all parts of the healthcare system. Structured, active recruitment to follow-up after hospital discharge and planned systematic outpatient visits, including support for home-to-clinic transport when required, could overcome physicians’ clinical inertia and facilitate the assessment of tolerability. Multidisciplinary teams including geriatric specialists, or dedicated cardiologists with a background in geriatrics, are needed to holistically approach the complexity of older patients, including management of multimorbid conditions and frailty. Referral to nurse-led clinics has been demonstrated to provide additional survival benefit in real-world practice, and this can be even reinforced in older age categories.50 Additional strategies, such as remote monitoring, home delivery of medications, and nursing support at home, could promote adherence to treatment and facilitate early variations and treatment intensifications to limit episodes of HF worsening. Socio-economic interventions are also part of the holistic care of older patients, who more frequently experience poor social and economic conditions. Consistent consideration of these different aspects may help achieve the complete implementation of HF treatments in older patients, with important consequences in terms of prognostic benefit. Finally, a more individualised approach could allow better tailoring of treatment strategies for individual patients, according to their needs and wishes, to balance quality of life and longevity.










