An estimated 38 million people worldwide have heart failure (HF), a clinical syndrome of impaired ventricular filling or ejection defined by fluid retention, effort intolerance and dyspnoea on exertion.1 Left ventricular ejection fraction (LVEF) drives the classification of HF subtypes – HF with reduced ejection fraction (LVEF ≤40%; HFrEF), mid-range (LVEF 41–49%), or HF with preserved ejection fraction (LVEF ≥50%; HFpEF) – because of differences in aetiology, responses to therapy and prognosis among them. Although HFpEF is the most common HF phenotype in the US and globally, the management of HFrEF has the most robust evidence base.2
Type 2 diabetes (T2D) is an increasingly common concurrent diagnosis with HFrEF. In total, 60% of Americans with HFrEF have demonstrated insulin resistance and more than 40% of patients with HFrEF carry a concomitant diagnosis of T2D.3 T2D and HF are also independent risk factors for one another. A diagnosis of HFrEF independently raises the incidence ratio of T2D by 2.5-fold over the general population.3 Patients with T2D have a two- to fourfold increased risk of HF, with each 1% increase in HbA1c portending an increase in incident HF by 8–36%.4–6 In addition, the two conditions independently worsen outcomes for one another.
T2D is an independent predictor of persistently unfavourable quality of life in patients with HFrEF.7 In individuals aged >65 years, the co-occurrence of HF and T2D increases mortality 10-fold over those without HF, and 1-year rates of all-cause death, in-hospital death and rehospitalisation for worsening HF are higher in patients with HFrEF and T2D than in those without T2D.8,9
Fortunately, recent trials of novel antidiabetic agents have demonstrated robust benefits on HF, independent of their impacts on glycaemic control. Therefore, it is imperative for clinicians treating HFrEF to become accustomed to the management of patients with concurrent T2D.
This review seeks to summarise contemporary data regarding available therapies for T2D as well as the evidence for benefit and harms of these therapies in patients with stage C HFrEF.
Glycaemic Goals in Stage C HFrEF
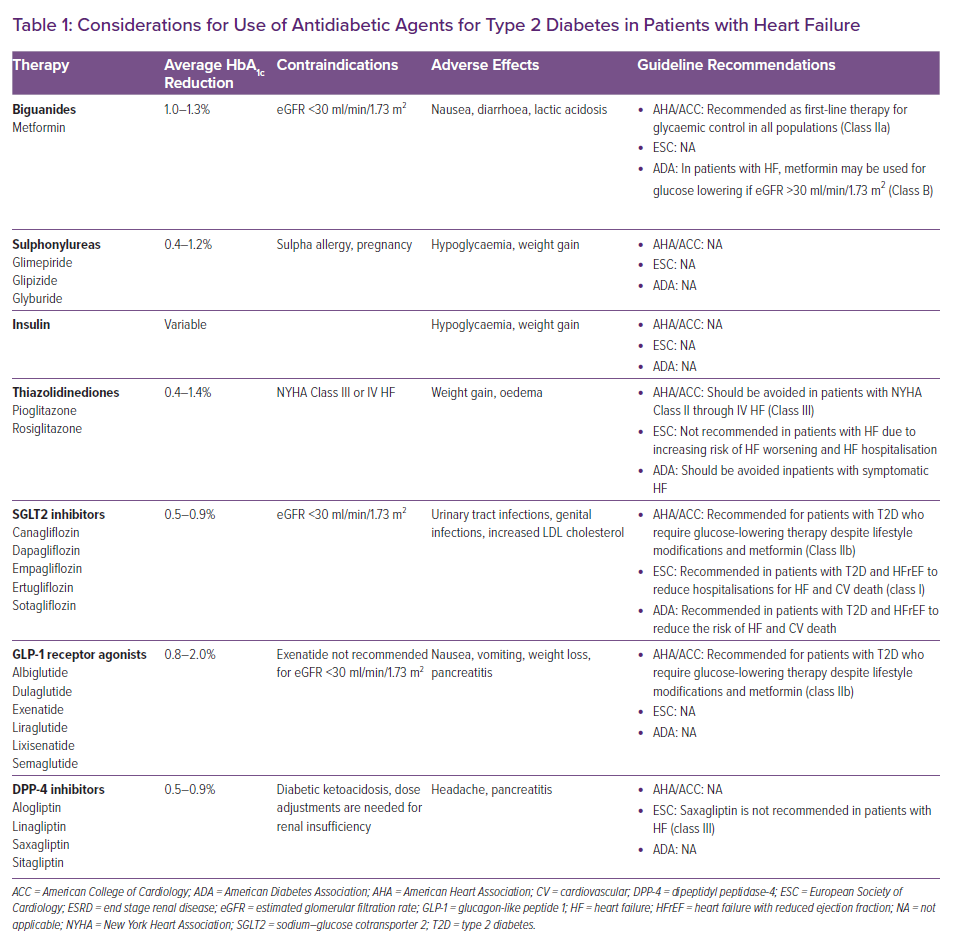
The 2021 American Diabetes Association (ADA) guidelines recommend a patient-centred approach to glycaemic goal setting.10 The 2019 guidelines developed by the European Society of Cardiology in conjunction with the European Association for the Study of Diabetes similarly posits a class IA recommendation for a target HbA1c <7% when possible, but with an individualised approach based on age, duration of T2D and comorbidities.11 For patients with pre-existing HF, there are minimal data to guide this approach. A U-shaped relationship between HbA1c and mortality in patients with concurrent HF and T2D has been suggested for ambulatory patients with HF and T2D, with the lowest risk of death at HbA1c between 7.1–7.8%.12 More data exist for the impact of glycaemic control in preventing incident HF. The UK Prospective Diabetes Study suggested that in patients with recently diagnosed T2D without HF, a 1% reduction in mean HbA1c was associated with a 16% decrease in incident H=F (95% CI [3–16%]; p=0.016).13 However, these results were not able to be replicated in patients with longer-term T2D diagnoses. In multiple large randomised trials, such as ACCORD, ADVANCE and VADT, intensive glycaemic control did not reduce the risk of HF.14,15,16
Altogether, a moderate approach to glycaemic control may be most appropriate in patients with pre-existing HF. The 2017 American College of Cardiology/American Heart Association (AHA)/Heart Failure Society of America guideline suggests an HbA1c target of 7–8% in patients with stage C HFrEF with individualisation to reflect comorbidity burden.17 In those with longer life expectancy, it is reasonable to pursue a target HbA1c of <7%. In those with limited life expectancy, the risks of symptomatic hypoglycaemia outweigh the benefit of intensive glycaemic control, especially as the majority of the benefit of intensive glycaemic control is based on long-term microvascular complications rather than short-term macrovascular ones.18
Antidiabetic Therapies with Proven Benefit in Stage C HFrEF
Sodium–Glucose Cotransporter 2 Inhibitors
In patients with HF, as well as in patients with high atherosclerotic cardiovascular (CV) disease risk or chronic kidney disease, sodium–glucose cotransporter 2 (SGLT2) inhibitors are recommended for glucose lowering independent of HbA1c or metformin use. Two drugs in this class, dapagliflozin and empagliflozin, have recently been added to the 2021 European Society of Cardiology-Heart Failure Association guidelines as class I, level A recommendation to reduce HF hospitalisations and deaths in all patients with HFrEF.19
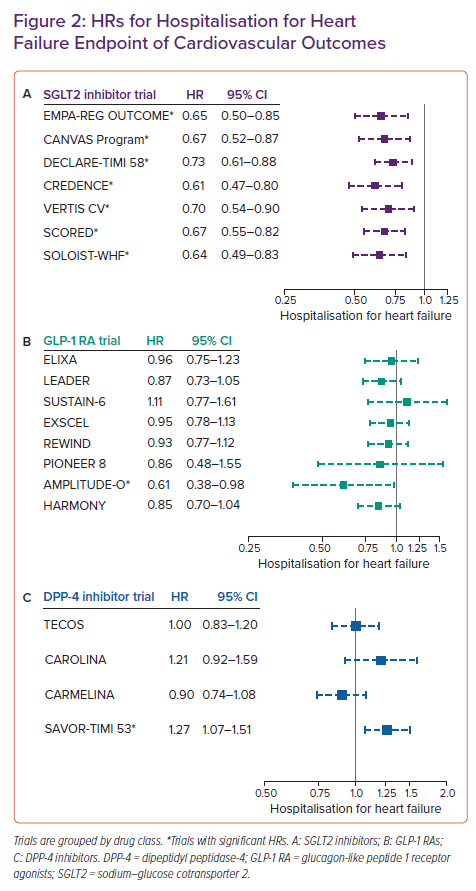
This recommendation is based on the class benefit of reduced major adverse cardiovascular events (MACE) and reduced composite CV death or hospitalisation for HF with SGLT2 inhibitors. Three trials of SGLT2 inhibitors, including empagliflozin (EMPEROR-Reduced), dapagliflozin (DAPA-HF), and sotagliflozin (SOLOIST-WHF), an SGLT2 inhibitor that also inhibits gastrointestinal SGLT1, in patients with HFrEF showed a 30–35% reduced risk of HF hospitalisations regardless of T2D status.20–22 Multiple meta-analyses of studies of SGLT2 inhibitor use in patients with HF have reproduced these results, demonstrating that SGLT2 inhibitor use is associated with an approximate 15% relative risk reduction in all-cause mortality and CV mortality and an approximately 30% reduction in the risk of HF hospitalisation.23–26 These benefits are seen across age, sex, ethnicity, renal function and HF functional class. The EMPA-RESPONSE AHF trial showed that in 80 patients randomised to empagliflozin versus placebo for 60 days, an SGLT2 inhibitor initiated during acute HF episodes led to reduced risk of the combined endpoint of in-hospital worsening HF, rehospitalisation for HF or death and had an acceptable safety profile.27 The EMPULSE trial investigated the safety profile and cardiovascular benefits of empagliflozin initiation during a hospitalisation for acute HF. This was the first major trial to evaluate inpatient initiation of an SGLT2 inhibitor and enrolled patients regardless of LVEF and diabetic status. In this population, treatment with empagliflozin led to decreased all-cause mortality, improved quality of life and greater decrease in body weight at 90 days compared to placebo with no associated safety concerns.28
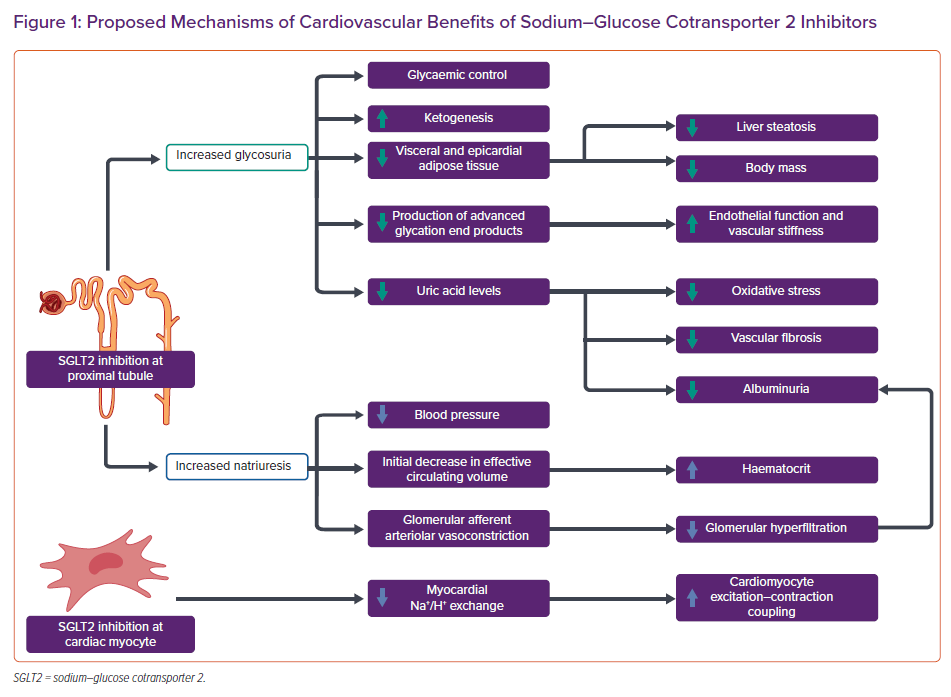
Though the mechanisms by which SGLT2 inhibitors provide these benefits in HF were not specifically studied in the above-mentioned CV outcomes trials (CVOTs), many possibilities have been proposed (Figure 1).29 These possible mechanisms include the direct benefits of SGLT2 inhibition at the proximal convoluted tubule of the glomerulus. SGLT2 inhibitors prevent the reabsorption of glucose through the SGLT2 cotransporter, which is typically responsible for 90% of the glucose reuptake at the level of the glomerulus. This allows for a reduction in plasma glucose levels through glycosuria.30 As such, hypoglycaemia is not a typical side effect of SGLT2 inhibitors, unlike other antidiabetic agents. This increased glycosuria also results in the loss of approximately 200–250 kcal per day, resulting in reduction in body mass.31 Additionally, SGLT2 inhibition prevents sodium reabsorption, resulting in an acute natriuretic effect before the kidney is able to re-equilibrate. This results in reductions in effective circulating volume, blood pressure and glomerular afferent arteriolar vasoconstriction.
Also cited are the less well-studied effects of SGLT2 inhibitors. These include their association with reduced adipose tissue deposition, as epicardial adipose tissue has been associated with increased risks of coronary artery disease, AF and cardiomyopathy.32–34 SGLT2 inhibition also promotes a metabolic shift from glucose oxidation towards ketone body production that may provide an energetic advantage to cardiac myocytes and may reduce the levels of hepatic and cardiac steatosis through increased lipid metabolism. Moreover, SGLT2 inhibitors have been shown to inhibit the myocardial sodium/hydrogen exchanger and potentially improve cardiac myocyte excitation–contraction coupling in animal models.29
Despite our incomplete mechanistic understanding of SGLT2 inhibition, the remarkable benefits of these drugs with their excellent safety profiles make them first-line agents for diabetic control in stage C HFrEF.
Metformin
Metformin is a biguanide that affects glycaemic control through three major mechanisms: decreased hepatic glucose production, decreased intestinal absorption of glucose and improved insulin sensitivity by increasing peripheral glucose uptake and use.35 As such, it does not impact insulin secretion and does not cause hypoglycaemia. It is recommended as first-line antidiabetic therapy in all populations with appropriate renal function (estimated glomerular filtration rate >30 ml/min/1.73 m2), regardless of HF status.
Within the first year of the approval of metformin by the Food and Drug Administration (FDA) in 1995, there was a notable incidence of lactic acidosis in HF patients receiving the drug, leading to contraindication labelling in this population.36 However, the FDA eliminated this contraindication following two large observational studies that demonstrated lower mortality and fewer hospitalisations with metformin use in HF patients compared with the use of sulphonylureas and thiazolidinediones.37,38 The mechanism behind this benefit is not well understood, though a potential pathway was proposed by a randomised placebo-controlled trial testing metformin treatment in insulin-resistant patients with HFrEF that showed a 20% improvement in myocardial efficiency via reduced myocardial oxygen consumption.39
Though no prospective randomised controlled trials (RCTs) examining metformin use in HF have been performed, numerous observational studies have replicated the findings of safety and lower mortality in metformin use in HF. In direct comparison with sulphonylurea monotherapy, metformin demonstrated lower morbidity and mortality over an average of 2.5 years when used alone or in combination.38 A 2007 systematic review of eight studies examining the use of available antidiabetic agents at the time that showed that metformin was the only drug not associated with harm in patients with HF and T2D.40
A retrospective cohort study of 6,185 patients treated for HF and T2D in Veterans Affairs clinics demonstrated that metformin therapy was associated with lower rates of mortality in ambulatory HF patients at 2-year follow-up.41 A 2013 meta-analysis of 34,000 patients with T2D and HF across nine cohort studies similarly showed metformin was associated with decreased risk of all-cause mortality and hospitalisations.42 A retrospective cohort study investigating associations between initiation of metformin and sulphonylurea and clinical outcomes among patients with comorbid HF and diabetes showed that metformin initiation was significantly associated with lower risk of composite all-cause mortality or HF hospitalisations at 12 months. However, this was primarily driven by impact on patients with LVEF >40%; when stratified for LVEF <40%, metformin initiation had no statistically significant impact upon HF hospitalisation or mortality.43
Overall, for patients with stage C HFrEF with appropriate renal function, metformin is a safe and effective first-line antidiabetic agent given its relatively benign safety profile and association with improved morbidity and mortality outcomes.
Antidiabetic Therapies to Be Used with Caution in Stage C HFrEF
Glucagon-like Peptide-1 Receptor Agonists
In the era of requiring CVOT for new glucose-lowering therapies, multiple society guidelines recommend SGLT2 inhibitors and glucagon-like peptide-1 receptor agonists (GLP-1 RA) as first-line anti-diabetic agents in patients with T2D and established CV risk, with a preference for SGLT2 inhibitors for those who have an existing diagnosis of HF.44 GLP-1 RAs work to enhance the action of endogenous GLP-1 on pancreatic cells to increase insulin secretion and decrease glucagon secretion. Currently available GLP-1 RAs include the twice-daily injectable exenatide; once-daily injectable liraglutide and lixisenatide; once-weekly injectable exenatide extended-release semaglutide and dulaglutide; and oral once-daily semaglutide. Albiglutide was permanently discontinued worldwide by its parent company in 2018 because of limited prescribing of the drug. Efpeglenatide and tirzepatide (a dual GLP-1 RA and glucose-dependent insulinotropic polypeptide receptor agonist) are not yet approved in the US or Europe.45–47
There are several CVOTs examining the effects of GLP-1 RA use in patients with T2D and varying levels of CV risk. However, none of the eight major GLP-1 RA CVOT to date (ELIXA, LEADER, SUSTAIN-6, EXSCEL, HARMONY, REWIND, PIONEER 6 and AMPLITUDE-O) included HF in the primary composite outcome.45,48–54 The proportions of trial participants with a history of HF at enrolment ranged from 8.6% in REWIND (dulaglutide) to 23.6% in SUSTAIN-6 (semaglutide). Information about participants’ HF subtype and baseline LVEF were not uniformly provided or standardised in these trials.
Hospitalisation for HF was examined as a separate secondary endpoint in ELIXA (HR 0.96; 95% CI [0.75–1.23]; p=0.75); LEADER (HR 0.87; 95% CI [0.73–1.05]; p=0.14); SUSTAIN-6 (HR 1.11; 95% CI [0.77–1.61]; p=0.57); EXSCEL (HR 0.95; 95% CI [0.78–1.13]); REWIND (HR 0.93; 95% CI [0.77–1.12]; p=0.46); PIONEER 6 (HR 0.86; 95% CI [0.48–1.55]); and AMPLITUDE-O (HR 0.61; 95% CI [0.38–0.98]) and as a composite secondary endpoint in HARMONY (HR 0.85; 95% CI [0.70–1.04]; p=0.113).45,48–54 Among these trials, AMPLITUDE-O was the only trial associated with a significant reduction in risk of HF hospitalisation. Recent meta-analyses of the major GLP-1 RA trials have demonstrated a 12% reduction in all-cause mortality and an approximately 10% reduction in HF hospitalisation with use of this drug class.55–57
However, little data regarding the use of GLP-1 RA in patients with established HF exist. Subgroup analyses of the aforementioned trials demonstrated no reduction in all-cause mortality in patients with HF.44 Post-hoc analysis of the SUSTAIN-6 and PIONEER 6 trials showed that semaglutide had no effect on MACE in patients with baseline HF.58 Trials specifically designed to target this question have enrolled relatively few patients. These include the LIVE trial, an RCT of 241 patients with chronic stable HFrEF randomised to placebo versus liraglutide for 24 weeks; and the FIGHT trial, an RCT of 300 patients with HFrEF with a recent decompensation randomly assigned to liraglutide or placebo for 6 months.59,60 In LIVE, there were no significant improvements in LVEF (p=0.24), quality of life (p=0.39) or functional class for those randomised to liraglutide. However, patients in the intervention arm were noted to have a mean 7 BPM increase in heart rate (p<0.0001), a finding which may be clinically significant as increased heart rate has been associated with worsened outcomes in HFrEF. Additionally, there were significantly more serious adverse cardiac events in patients on liraglutide than on placebo (10% versus 3%, p=0.04), including sustained ventricular tachycardia, AF requiring intervention and aggravation of ischaemic heart disease. In FIGHT, there were again no differences in HF-related outcomes or functional capacity; however, there was a signal for increased composite outcome of death and HF hospitalisation in patients on liraglutide.
As such, in patients with pre-existing HF, GLP-1 RA may not offer the same cardioprotective effects compared with those in patients without HF and may have a worse safety profile in this population. Further inquiry into the clinical significance of these findings is necessary before incorporation of GLP-1 RA into standard therapy for patients with diabetes and HFrEF.
Sulphonylureas
Sulphonylureas, such as glipizide and glimepiride, increase insulin release from pancreatic β-cells. There are no RCTs that examine sulphonylurea use in HF. A cohort study in the Veterans Affairs system showed a higher risk of HF and CV death with sulphonylurea use compared with metformin, but this was likely confounded by indication as those who could not take metformin due to comorbidities were more likely to be on sulphonylureas.61 A retrospective cohort study of clinical outcomes following sulphonylurea initiation in patients with HF and concurrent diabetes showed that sulphonylurea initiation was associated with a nominally statistically significant excess risk of all-cause mortality (HR 1.24; 95% CI [1.00–1.52]; p=0.045). Initiation was also associated with higher risk of composite all-cause mortality and HF hospitalisation (HR 1.17; 95% CI [1.00–1.37]; p=0.047) and HF hospitalisation alone (HR 1.22; 95% CI [1.00–1.48]; p=0.050). This was consistent when stratified for LVEF >40% and <40%.43 Given the lack of data of any demonstrable benefit, sulphonylureas are not recommended as initial therapy in patients with stage C HFrEF.
Insulin
Insulin is the main anabolic hormone that promotes glucose uptake into the liver, adipose and skeletal muscle tissue and was the original mainstay of diabetes management. Cardiac insulin resistance has become an increasingly recognised factor in the development of HF. As cardiac myocytes develop insulin resistance, they become metabolically inflexible, resulting in energy deficiency which further leads to diastolic dysfunction, myocardial cell death and fibrosis.62 Hyperinsulinaemia has also been implicated in worsening HF, through progressive insulin resistance and oxidative stress, increased obesity and fluid retention.
In patients with established HF, there is little evidence about the safety and efficacy of insulin use. No RCTs have been conducted with insulin use in patients with HF, and observational studies are often confounded by indication, as insulin is more likely to be used in patients with more advanced T2D or severe comorbidities. The ORIGIN trial showed no impact of basal insulin glargine on CV outcomes and HF events in patients with CV risk factors in comparison to standard of care.63 Given insulin’s correlation with hypoglycaemia and weight gain and lack of demonstrable CV benefit with use, other agents, such as metformin and SGLT2 inhibitors should be used preferentially unless appropriate glycaemic control cannot be achieved solely with these agents.
Antidiabetic Therapies to Avoid in Stage C HFrEF
Thiazolidinediones
Thiazolidinediones act on PPAR-γ, a nuclear transcription regulator, to increase insulin action and insulin sensitivity in muscle and fat and decrease hepatic gluconeogenesis. A 2003 consensus statement from the AHA and ADA recommended that thiazolidinediones be avoided in patients with New York Heart Association (NYHA) class III and IV HF and used with caution in those with class I or II symptoms.64 This statement was in response to reports of increased fluid retention with thiazolidinedione use. A meta-analysis by Lago et al. showed a 1.7-fold increase in the relative risk of HF with thiazolidinedione use (95% CI [1.2–2.42]; p=0.002), with slightly greater risk with rosiglitazone than pioglitazone.65 Lincoff et al. similarly demonstrated a 1.4-fold increase in the risk of HF with pioglitazone (95% CI [1.14–1.76]; p=0.002).66 The RECORD study showed that addition of rosiglitazone to metformin or a sulphonylurea significantly increased the risk of HF death or hospitalisation (HR 2.1; 95% CI [1.30–3.27]; p=0.001).67 A 2007 Canadian meta-analysis of three RCTs and four observational studies estimated a number needed to harm of approximately 50 over a 2.2 year period (OR 2.10; 95% CI [1.08–4.08]; p=0.03).68
Of note, these studies did not show an increase in the risk of CV death. In a 2007 RCT of 224 patients with T2D and NYHA class I and II HF were randomised to 52 weeks of treatment with rosiglitazone versus placebo, the rosiglitazone group had improved glycaemic control without adverse impact on LVEF.69 While more congestion-related events occurred with thiazolidinedione treatment, the events generally did not result in study withdrawal and were managed with diuretics. As such, the clinical significance of the fluid retention with thiazolidinedione use is not well-defined. This is especially notable as the PROactive study, an RCT of over 5,000 patients with T2D comparing pioglitazone use versus placebo, demonstrated that pioglitazone is associated with reduced all-cause death, non-fatal MI and non-fatal stroke without difference in mortality.70 Overall, though the fluid retention associated with thiazolidinediones may not have long-term mortality consequences, thiazolidinediones should not be prescribed to patients with stage C HFrEF given the availability of medications with more definitively favourable safety and efficacy profiles.
Dipeptidyl Peptidase-4 Inhibitors
Dipeptidyl peptidase-4 (DPP-4) inhibitors are incretin mimetics. They act by inhibiting DPP-4, an enzyme that limits insulin release by inactivating incretins such as GLP-1 and gastric inhibitory polypeptide. This medication class has a mixed evidence base in HFrEF. The FDA recommends discontinuation of saxagliptin and alogliptin in patients who develop HF, supported by evidence from SAVOR-TIMI 53, which demonstrated a 27% increased risk of HF hospitalisations in patients receiving saxagliptin versus placebo (95% CI [1.07–1.51]; p=0.007).71
However, the EXAMINE trial was more equivocal, showing no statistically significant increase in the risk of HF hospitalisations in all patients (HR 1.07; 95% CI [0.79–1.46]). In a post-hoc subgroup analysis, when stratified for previous history of HF, treatment with alogliptin in those without a previous history of HF was associated with increased risk of HF hospitalisation (HR 1.76; 95% CI [1.07–2.90] p=0.026). Conversely, in patients with an established history of HF, there was no statistically significant increase in hospitalisation for HF (HR 1.00; 95% CI [0.71–1.42]; p=0.996). There was no significant interaction between treatment and history of HF (p=0.068).72
Additionally, this does not appear to be a class-wide effect. A 2015 RCT randomising 14,671 patients with established CV disease to adding sitagliptin or placebo to their existing therapy showed no increase in MACE or hospitalisation for HF in the intervention arm.73 The CARMELINA trial, a multicentre study of 6,979 patients with and without a history of HF, showed no increased incidence of HF hospitalisations over the 2.2-year follow up period, regardless of LVEF.74
The mechanism by which saxagliptin and alogliptin impart a seemingly greater risk of HF is largely unknown. Regardless of the demonstrated safety of other members of the DPP-4 inhibitor class in HF, their relatively high cost and the lack of strong CV benefit make metformin and SGLT2 inhibitors comparatively superior options. Additionally, saxagliptin should be avoided in patients with HF and clinicians should be aware of the risk of HF in patients taking alogliptin.
Considerations for the use of antidiabetic agents for patients with T2D and HF are summarised in Table 1. HRs for the endpoint of hospitalisation for HF in CVOTs of SGLT2 inhibitors, GLP-1 receptor agonists and DPP-4 inhibitors are shown in Figure 2.
Anticipated Trials in Diabetes and Heart Failure
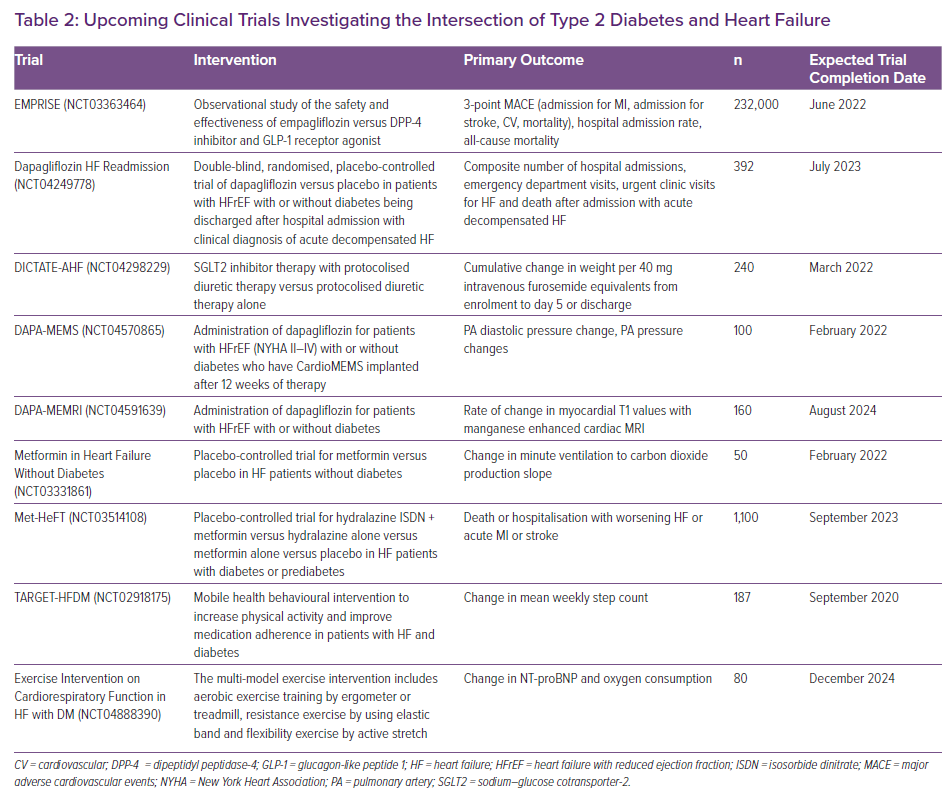
Many on-going and future trials aim to better elucidate the safety and efficacy of the newer antidiabetic therapies, while others plan to study available drugs to better understand the physiological interplay between T2D and HFrEF (Table 2). The majority of SGLT2 inhibitor CVOTs to date have enrolled patients with chronic, stable disease who are on optimal guideline-directed medical regimens for HF.20,21 Upcoming trials will further investigate the effect of SGLT2 inhibitor initiation in acute HF. The Dapagliflozin HF Readmission trial will investigate whether initiating dapagliflozin following a HF admission affects subsequent admissions, urgent care visits, and mortality for patients with HF (NCT04249778). The DICTATE-AHF trial will test the efficacy of dapagliflozin along with protocolised diuretic therapy during hospitalisation for acute HF in patients with T2D (NCT04298229). Other studies will use observational data to examine the real world efficacy and safety of SGLT2 inhibition. The EMPRISE study, for example, is comparing CV endpoints for over 230,000 people with T2D who take empagliflozin, DPP-4 inhibitors or GLP-1 RAs (NCT03363464).
In addition to studying the timing of initiation and efficacy of SGLT2 inhibitor therapy, imaging and invasive strategies are being employed to better understand the pleotropic mechanisms of benefit. The DAPA-MEMRI study investigators theorise that dapagliflozin improves calcium-handling in patients with HF, and enrolled patients will be randomised to receive dapagliflozin or placebo with manganese-enhanced cardiac MRI at baseline and 6 months to quantify the potential mechanistic benefit of SGLT2 inhibition in HF (NCT04591639). Investigators of the DAPA-MEMS trial plan to utilise the CardioMEMS device to study invasive pulmonary pressure measurements in ambulatory patients. This trial aims to study changes to pulmonary artery pressures, diuretic dosing and functional capacity for patients with HFrEF and a CardioMEMS sensor after 12 weeks of therapy with dapagliflozin (NCT04570865).
Other studies are examining whether available antidiabetic agents may also positively affect cardiac function. The MEASURE-HF trial is investigating whether the DPP-4 inhibitors saxagliptin and sitagliptin improve cardiac dimensions and function in patients with T2D and HF (NCT02917031). In a pilot study called Metformin in Heart Failure Without Diabetes, patients with HF but without diabetes will be randomised to receive metformin or placebo and will receive on-going functional testing and follow-up for HF symptoms and hospitalisation (NCT03331861). A similar study from the Danish Heart Foundation plans to test the efficacy and safety of metformin for patients with HFrEF and a diagnosis of diabetes or prediabetes (Met-HeFT; NCT03514108). With an estimated 1,500 participants, this will be the largest prospective RCT to date testing the recommendation that metformin should be first-line therapy for most patients with diabetes and HF.75
Finally, there are on-going trials that focus on non-pharmacological interventions for patients with HF and diabetes. The TARGET-HFDM trial will leverage consumer mobile health technology to improve medication adherence and physical activity in subjects with HF and diabetes (NCT02918175). Another trial will test whether a multi-model exercise programme will affect cardiorespiratory function and exercise capacity in patients with HF and diabetes (NCT04888390). The majority of these studies have target end-dates in 2022–2024, highlighting the rapidly evolving landscape of therapies for patients with diabetes and HF.
Guideline-directed Medical Therapy for Heart Failure in Patients with Diabetes
There are no RCTs assessing the efficacy of guideline-directed medical therapy (GDMT) of HFrEF specifically in patients with diabetes. However, enrolled participants in the original landmark trials had a high prevalence of diabetes, allowing for many subsequent subgroup analyses. The evidence suggests consistent benefits of GDMT when comparing patients with and without diabetes. As a result, the general recommendations for HF management remain the same regardless of diabetes status.17,76 Detailed landmark trial outcomes and diabetes status treatment effects are summarised in Supplementary Material Table 1.
Conclusion
Individuals with concomitant T2D and stage C HFrEF have an increased risk for adverse cardiovascular outcomes, and aggressive cardiometabolic risk factor modification is imperative to combat the growing global burden of these diseases. The past decade has seen a paradigm shift in the management of these comorbid diseases with a proliferation of novel antidiabetic agents with broad cardiovascular benefits. Current data emphasise that for patients with stage C HFrEF, clinicians should aim for moderate glycaemic control with an HbA1c target 7–8%, guided by the clinical context and life expectancy of a patient. To achieve this goal, the drugs with the strongest evidence for safety and CV benefit are metformin and SGLT2 inhibitors and these agents should be used as first-line therapies in this comorbid population. Thiazolidinediones and saxagliptin should be avoided given their risks of worsening HF.
There remains a need for rigorous CVOTs with diverse participant enrolments to guide use of insulin, GLP-1 RA, sulphonylureas, DPP-4 inhibitors to advance the care of patients with T2D and stage C HFrEF.