Cardiovascular sequelae of COVID-19 include venous and arterial thrombosis, electrical disturbances, and mechanical dysfunction.1–3 As many as 55% of patients with acute COVID-19 have cardiovascular abnormalities detected by echocardiography, and elevations of troponin I and N-terminal pro B-type natriuretic peptide during acute, severe illness are predictors of mortality.4,5 However, among ambulatory patients, traditional cardiovascular diagnostics, such as echocardiography with strain imaging, troponin I and electrocardiography, perform inconsistently as screening tools for COVID-19-related myocarditis.6–8
Myocardial oedema, inflammation and fibrosis have been detected by cardiac magnetic resonance (CMR) in both the acute and late convalescent phases of COVID-19 illness.7–9 Indeed, these cardiovascular sequelae have consistently been detected by CMR among competitive athletes recovering from COVID-19 in the setting of normal echocardiography.6–7 Pathological analysis of the myocardium following COVID-19 remains the gold standard for the diagnosis of direct and indirect myocardial injury, however endomyocardial biopsy is invasive and has limited sensitivity.10 One autopsy study involving 15 patients who had died of COVID-19 revealed nonocclusive fibrin microthrombi in the coronary arteries of 80% (n=12), and active lymphocytic myocarditis in one-third (n=5). No viral particles were identified in the cardiac myocytes, vascular endothelium or interstitial fibroblasts, suggesting an indirect inflammatory process rather than a direct viral invasion of the myocardium.11
CMR has distinct advantages for cardiovascular phenotyping. CMR is not only the reference standard for the assessment of ventricular volumes and function but it can be used to diagnose subclinical myocardial dysfunction using strain analysis. However, its biggest advantage over other imaging modalities is advanced tissue characterisation. Late gadolinium enhancement (LGE) imaging can be used to identify focal areas of oedema and replacement fibrosis, and parametric mapping is extremely sensitive for the detection of diffuse oedema, inflammation, fibrosis, infiltration, and fat. This has made CMR a critical tool for clinicians to understand cardiovascular involvement and for risk stratification of patients recovering from COVID-19.
Advantages of Cardiac Magnetic Resonance
Volumes and Function
CMR is the gold standard for assessment of myocardial volumes and function.12 Accurate quantitative assessment of biventricular function, cardiac performance and valvular disease can be reliably obtained with CMR. CMR is particularly beneficial for the accurate assessment of right ventricular (RV) size and function, and multiple studies have shown a reduced RV ejection fraction among patients recovering from COVID-19 compared to controls.9,13,14
Strain
Myocardial strain imaging with CMR may enhance the detection of subclinical functional abnormalities of the myocardium after COVID-19. Strain imaging adds incremental prognostic value to predict major adverse cardiac events (MACE) in comparison to traditional CMR in acute myocarditis.15,16 In particular, CMR strain has been shown to improve diagnostic accuracy in the setting of preserved biventricular systolic function.17–19 Given that patients with COVID-19-related myocarditis tend to have normal biventricular systolic function, investigations of CMR strain may provide further insights into subclinical cardiovascular sequelae after recovery from COVID-19.7,8
Parametric Mapping
Native T1, T2 and extracellular volume (ECV) mapping by CMR can be used for myocardial tissue characterisation, specifically to detect myocardial inflammation, oedema and fibrosis.20 The modified Lake Louise criteria for the detection of myocarditis include the use of these advanced parametric mapping techniques for improved sensitivity.21 Elevation of the native myocardial T1 is non-specific and may be due to any combination of inflammation, oedema, injury and/or infiltration. T2 elevations correspond to myocardial oedema. The resolution of myocardial oedema with persistence of LGE is known to be an unfavourable prognostic marker in viral myocarditis.22 ECV elevations suggest extracellular compartment expansion. Accurate ECV calculation requires a recent haematocrit. Native T1 and T2 mapping values depend on the magnetic field strength and other unique properties of the individual magnet, pulse sequences and field inhomogeneities in the field of view. Thus, reference to internal magnetic-specific control values is preferred for native T1 and T2 mapping. On the other hand, ECV is derived from the ratio of native and post-contrast T1 values and is therefore similar between different magnets, allowing for the use of published controls.20
Late Gadolinium Enhancement
Contrasted CMR permits the assessment of myopericardial LGE. The presence, location and extent of LGE relative to the ventricular mass have been shown to be associated with MACE in patients with myocarditis.23–25 The distribution of LGE aids the differentiation of ischaemic versus non-ischaemic complications of COVID-19. LGE burden in viral myocarditis often decreases over time with acute phase LGE with myocardial oedema representing inflammation and extracellular expansion, while late phase LGE reflects replacement fibrosis.22,26
Stress Imaging
Vasodilator stress CMR is a highly accurate and useful diagnostic modality for patients with suspected or known coronary artery disease (CAD) and post-orthotopic heart transplantation to assess for coronary allograft vasculopathy.27–31 Because 95% of coronary arterial blood supplying the myocardium returns through the coronary sinus (CS), flow through this vessel is an accurate surrogate for total coronary blood flow.29 The coronary flow reserve (CFR) may be calculated as the ratio of CS flow during vasodilator infusion versus resting conditions. An impaired CFR may indicate either obstructive CAD or coronary microvascular dysfunction.
An autopsy study of COVID-19 patients found an absence of viral particles in the myocardium and thrombosis of the coronary microvasculature during the acute and convalescent stages. This suggests that COVID-19-mediated myocardial injury and ischaemia may result from endothelial injury and demand ischaemia from microthrombi.11 COVID-19-related microthrombi and endothelial injury may therefore be detectable by CFR assessment from stress CMR. Thus, stress CMR with CFR may be uniquely suited to explore this mechanism of cardiovascular involvement after COVID-19 and may yield insights into patients with cardiovascular post-acute sequelae of COVID-19 (CV-PASC).
Unique Populations
Competitive Athletes and Military Personnel
Myocarditis is a known complication of COVID-19 and a leading cause of sudden cardiac death (SCD) among athletes and military recruits in the US.32 The detection of myocarditis in athletes presents unique challenges owing to the heterogeneity of clinical presentation, lack of specific biomarkers for early detection, underlying structural myocardial changes related to dynamic exertion, uncertainty about the true impact of exercise on SCD risk, and downstream training and career implications for athletes who are restricted from activity.33 While the pathophysiology of myocardial injury from COVID-19 is debated, myocarditis remains a primary consideration in decisions to return-to-play among athletes and sports cardiologists.
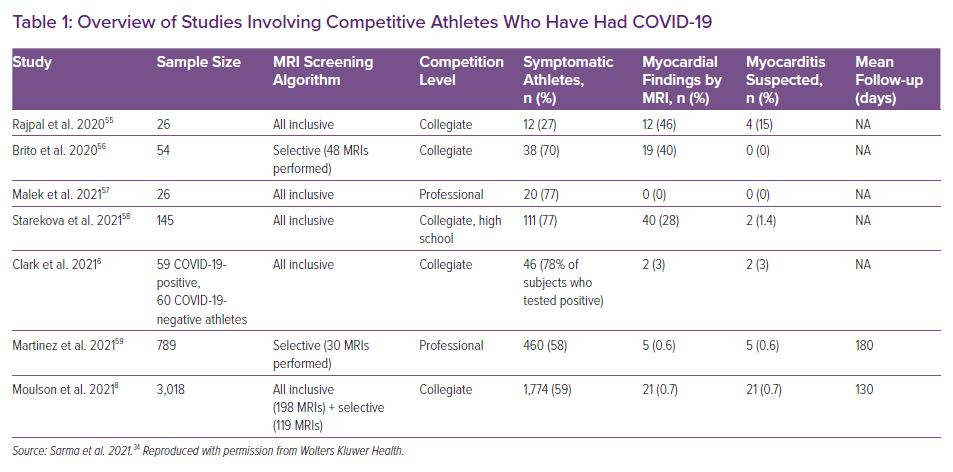
Contrasted CMR with parametric mapping is uniquely suited as a tool for detection of cardiovascular complications from COVID-19 for the reasons outlined above and has quickly and widely been deployed to understand the cardiovascular complications of COVID-19. Table 1 summarises the findings of key CMR studies among competitive athletes recovering from COVID-19.34 Combined single-centre and multicentre data have shown that the prevalence of myocarditis among competitive athletes is approximately 3% when universal screening with CMR is undertaken (Table 1).
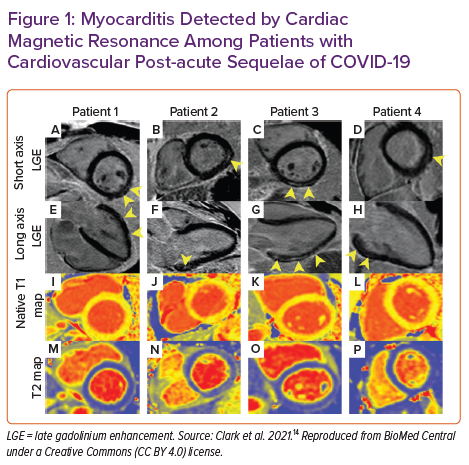
Cardiovascular Post-acute Sequelae of Coronavirus Infection
A study of military personnel with CV-PASC found a higher rate (12%) of myocardial pathology – mostly myocarditis – among this symptomatic cohort.14 For high-performance athletes, employees in high-risk professions (pilots, military personnel and other high-stake professions in which arrhythmias and/or SCD have implications beyond the person affected), and people with underlying cardiovascular abnormalities that make superimposed post-COVID-19 symptoms difficult to discriminate, we propose CMR with parametric mapping with or without stress testing as a crucial tool to improve detection of pathology or provide reassurance of its absence (Figure 1).
Multisystem Inflammatory Syndrome
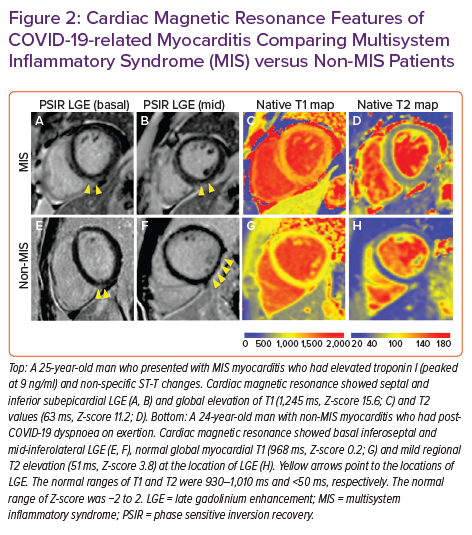
Multisystem inflammatory syndrome (MIS) has now been reported in both children and adults following infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).35,36 Cardiovascular involvement in MIS is variable, with presentations ranging from myocarditis, pericarditis, transient systolic dysfunction, arrhythmias, coronary artery ectasia/aneurysms and cardiogenic shock.37,38 An inflammatory cardiomyopathy with global elevations in T1 and T2 values has been the characteristic finding in a limited series of MIS patients undergoing CMR, often with mild or no myocardial LGE (Figure 2).39–42 Although echocardiography is often normal, especially after recovery from acute COVID-19, CMR reveals subclinical ventricular dysfunction – evidenced by abnormal strain – that persists as inflammation resolves.40,41 Follow-up CMR in small cohorts of patients reveal that MIS often presents as a transient inflammatory cardiomyopathy, with no residual cardiac abnormalities identified a few months after diagnosis.43,44 Thus, CMR may be a useful tool during the acute phase of MIS to assist with cardiovascular phenotyping and post-acute MIS to identify residual myocardial pathology and resolution of cardiac inflammation.
Vaccine-associated Myocarditis
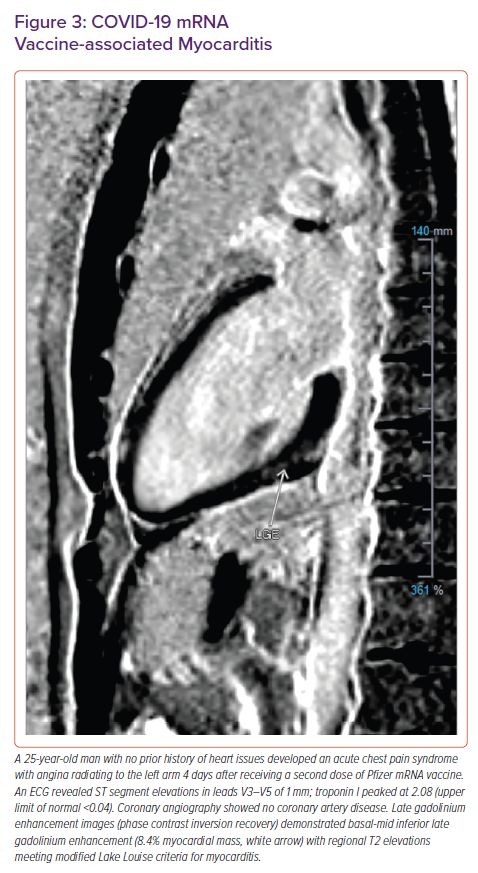
Vaccine-associated myocarditis is not a new entity, having previously been reported in 7.8 per 100,000 military service members within 30 days of smallpox vaccination.45 Early reports suggest that vaccine-associated myocarditis is a rare complication of the mRNA COVID-19 vaccine and young men may be at higher risk. Most affected patients present with an acute chest pain syndrome and biomarker evidence of myocardial injury within a week of the second dose of the mRNA vaccine.46–48 These preliminary reports show an incidence of less than 0.001% in comparison to the approximate 3% rate of myocarditis after COVID-19 in competitive athletes. In our experience, mRNA COVID-19 vaccine-associated myocarditis is associated with preserved biventricular systolic function and a modest LGE burden and is similar in appearance to acute COVID-19-related myocarditis (Figure 3). Thus, while vigilance for this rare adverse event is advised, there appears to be a much higher likelihood of myocarditis in the acute and convalescent phases after COVID-19 than following mRNA COVID-19 vaccination.
Challenges Associated with CMR
Limitations of CMR
CMR is limited by availability, expertise, time constraints (as comprehensive examinations may take more than an hour at some centres) and patient-specific factors, such as claustrophobia, retained leads or non-compatible devices. Despite the common misperception, cost should not be a significant barrier to CMR. In the US, reimbursement for CMR (US$570 for a CMR with contrast) from the Centers for Medicare and Medicaid Services is typically less than single photon emission CT and only slightly greater than echocardiography.49,50 The effective cost of CMR is further diminished after accounting for the cost of downstream consequences of not carrying out these examinations.50
CMR Interpretation Challenges Specific to COVID-19
The Society for Cardiac Magnetic Resonance recommends standardised reporting of parametric mapping in reference to magnet-specific reference values, which often have age- and gender-based normative ranges.20 However, not all centres who are able to offer CMR have collected magnet-specific control data and there are no guidelines to define appropriate selection of regions of interest (ROIs) to define regional parametric abnormalities. While parametric mapping is a highly sensitive technique for the detection of myocardial fibrosis/inflammation, interpretation of these images requires significant experience and may be subject to high inter-observer variability. This variability in parametric ROI reporting may account for some of the wide distribution of prevalence rates of myocarditis after COVID-19 detected by CMR.9
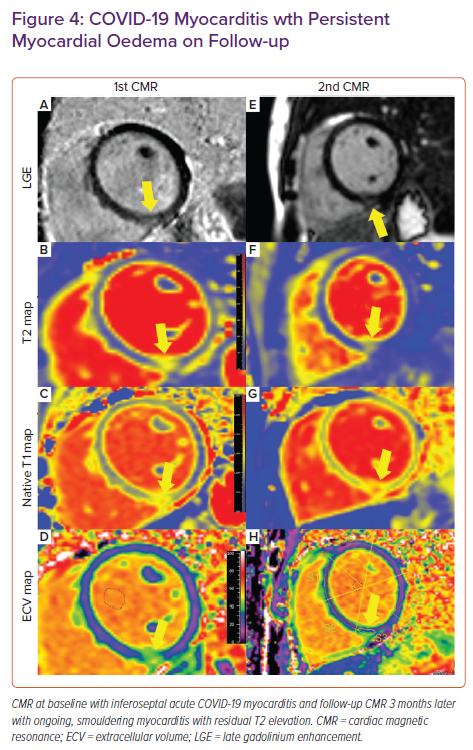
Additionally, interpretation of CMR images may be challenging owing to an apparent predilection of COVID-19 myocarditis to affect the inferior wall and inferoseptum near the RV septal insertion site (Figure 4).6,51 Focal LGE at the inferior RV septal insertion is common among athletes, which may lead to a tendency to over-diagnose pathology in this region without selection of a proper control group.6,52
Furthermore, LVEF tends to be preserved in this population, thereby making it more difficult to discriminate pathological LGE from non-pathological LGE at this location – especially in the cohort of athletes. All these factors may complicate the accurate diagnosis of myocarditis.
Recommendations for CMR Interpretation
The parametric mapping consensus guidelines recommend the following to standardise parametric mapping use:
- Use magnet-specific age- and gender-assigned controls with normal ranges defined by ±2 standard deviations from the mean.
- Perform basal- and mid-LV short axis mapping along with a four-chamber view for T1, T2 and ECV maps.
- Carefully review the data to ensure adequate motion correction and absence of artefact.
We propose the following additional measures to specifically address issues associated with the interpretation of CMR post-COVID-19:
- Report ROIs that represent no less than a half of a standard segment in the American Heart Association’s 17-segment model.53
- Report methodology, location and value of parametric mapping abnormalities in scientific publications.
We suggest the following characteristics to diagnose pathological LGE in COVID-19 myocarditis:
- LGE encompassing greater than 50% of myocardial thickness.
- LGE extending to at least two short-axis slices.
- Associated regional elevations in T2.
- Segmental hypokinesis.
The first two criteria should always be met; the latter two may help with diagnostic confidence (as a regionally normal T2 in the area of LGE may indicate healing myocarditis). The modified Lake Louise criteria require global or regional T1 and T2 abnormalities to diagnose acute myocarditis by CMR.21
Future Directions
Comparison to an appropriate control group has been shown to be of critical importance to contextualise CMR findings in COVID-19 and remains a crucial component of ongoing research.6,54 Further investigation is necessary to better understand the utility of CMR for return-to-play screening of competitive athletes and military personnel after COVID-19. Research assessing the clinical yield of CMR for patients in the late convalescent phase of recovery from COVID-19 with CV-PASC will provide insights into the prevalence of detectable myocardial structural, functional and tissue-level changes and their correlation with patient-reported symptoms.
Future studies correlating biomarkers of effector immune response, antigen-presenting cells, cytokines, antibodies and SARS-CoV-2 titres with comprehensive CMR may elucidate which features of COVID-19 and host immune response predispose to the greatest degree of myocardial inflammation and injury. Additionally, large follow-up studies are necessary to understand the clinical significance of subclinical CMR-based myocarditis findings. These analyses should include the following clinical outcomes: quantification of arrhythmia burden, quantification of functional limitations with cardiopulmonary exercise treadmill testing, heart failure, SCD and all-cause death. As the SARS-CoV-2 virus mutates and the pandemic evolves, studies will need to be conducted among different viral variants and in various phases of recovery (acute and chronic convalescent, PASC and late post-recovery). Multicentre collaborations will be necessary to understand the prevalence of smouldering myocarditis and exploring treatment options for this subset of patients.
Conclusion
Cardiovascular involvement following COVID-19 is prevalent and heterogenous in manifestation. CMR accurately diagnoses myocardial inflammation/injury and is the reference standard for the assessment of myocardial structure, function, tissue characterisation and perfusion. CMR should be considered for patients at heightened risk of COVID-19 complications and when the detection of subclinical myocardial inflammation will change medical management or restrict activity. CMR complications after COVID-19 may be subtle and normal findings in athletes may be misconstrued as pathology. Thus, expertise in the acquisition and interpretation of images is critical for accurate diagnosis of acute and post-COVID-19 complications by CMR. Future studies are necessary to determine the long-term implications of myocardial inflammation and injury detected by CMR after recovery from COVID-19.
Clinical Perspective
- Cardiovascular involvement following COVID-19 is prevalent and heterogenous in manifestation.
- Cardiac magnetic resonance (CMR) is uniquely suited to comprehensively phenotype high-risk, persistently symptomatic, or otherwise complicated patients who have had COVID-19.
- CMR following COVID-19 may be best suited for specific populations, such as athletes, patients who develop multisystem inflammatory syndrome, and those with suspected COVID-19 vaccine-induced myocarditis.
- Expertise and consistency in image acquisition, analysis, interpretation and reporting is especially critical in the diagnosis of myocarditis by CMR.