It is estimated globally that 2.41 billion, or one in three people, are in need of rehabilitation at some point.1 Of these, 37 million people are estimated to have cardiovascular disease, the vast majority (35 million) of whom have heart failure (HF), a leading cause of hospitalisation that is associated with poor quality of life, frequent re-hospitalisations, and high mortality and healthcare costs.2 Exercise-based cardiac rehabilitation (CR) – a comprehensive programme that includes exercise training, education, lifestyle modification and psychosocial support – is a safe and effective intervention that results in improvements in health-related quality of life, functional capacity, hospitalisation and morbidity, is cost-effective and a recommended treatment for a range of cardiac conditions, including HF.2,4–7 For example, in the 2021 European Society of Cardiology (ESC) guidelines on cardiovascular disease prevention in clinical practice, one of the recommendations (class 1, level a) states “Participation in a medically supervised, structured, comprehensive, multidisciplinary exercise-based CR (EBCR) and prevention programme for patients after atherosclerotic cardiovascular disease (ASCVD) events and/or revascularisation, and for patients with HF (mainly HFrEF), is recommended to improve patient outcome.” (p. 3308).7 The guidelines also provide recommendations for nutrition and alcohol, body weight, mental healthcare and psychosocial interventions, smoking intervention strategies and lifestyle interventions for hypertension. The 2021 ESC guidelines for the diagnosis and treatment of acute and chronic HF note that there is consistent evidence to show that physical conditioning by exercise training improves exercise tolerance and health-related quality of life in patients with HF, and mention the importance of exercise-based CR, but make recommendations for exercise rehabilitation in patients with chronic HF only, stating that “Exercise is recommended for all patients who are able in order to improve exercise capacity, QOL (quality of life), and reduce HF hospitalisation (Class I, Level A),” and “A supervised, exercise-based, cardiac rehabilitation programme should be considered in patients with more severe disease, frailty, or with comorbidities (Class IIa, Level c)” (p. 3636).2 These guidelines also recommend that patients with chronic HF receive patient education, self-care and lifestyle advice (p. 3636). The 2022 American Heart Association/American College of Cardiology/Heart Failure Society of America (AHA/ACC/HFSA) guideline for the management of HF is broadly similar, although it refers to stage C HF, and recommends, “For patients with HF who are able to participate, exercise training (or regular physical activity) is recommended to improve functional status, exercise performance, and QOL” (class 1, level a), and “In patients with HF, a cardiac rehabilitation program can be useful to improve functional capacity, exercise tolerance, and health-related QOL” (p. e32).8 However, despite this, only one-tenth of eligible HF patients receive CR referral at discharge and are thus deprived of its potential benefits.9
Exercise
Exercise rehabilitation has a long history in the management of chronic HF, with the first randomised trial of exercise training being published in 1990 and exercise training becoming an integral part of therapy a decade later.10–12 Although it is evident that exercise training improves exercise capacity, neurohormonal modulation (mainly by reducing N-terminal pro-B-type natriuretic peptide level)3 and quality of life, and reduces symptoms in people with stable HF, its effects on mortality are unclear.13,14 And although trial-level and individual participant meta-analyses demonstrate a reduction in both all-cause and HF hospital admission with rehabilitation, the lack of a prognostic benefit of rehabilitation is a real problem as patients take their poly-pharmaceutical treatment to improve their prognosis. There is also a dearth of studies examining the effects of exercise training in people who are old, frail or who have multiple conditions, that is, those in greatest need. An exception is the recent Rehabilitation Therapy in Older Acute Heart Failure Patients (REHAB-HF) trial of a transitional, tailored, progressive rehabilitation intervention based on patients’ individual abilities.15 The trial population had a mean age of 73 years and consisted mostly of frail or pre-frail patients with an average of five coexisting conditions. The intervention, focusing on four physical function domains (strength, mobility, balance, endurance) and progressing through four pre-specified functional levels in each domain, was commenced during, or early after, hospitalisation and was continued in 36 outpatient sessions, 60 minutes each, for 3 days per week for 12 weeks. Results included significant differences in physical performance (the primary outcome), depression and quality of life in favour of the intervention group.15 A secondary analysis of the trial suggests that longer term benefits of the intervention, particularly in patients with preserved ejection fraction, may yield good value to the healthcare system.16 However, implementing this intervention in the real world could be challenging, given that patients have to attend a centre and the healthcare system will require enough trained healthcare professionals to deliver and supervise the sessions.
Depression
The findings from the REHAB-HF trial indicate that exercise may result in improvements in mood, including a reduction in the feeling of depression, which is of note because depression is a common and significant comorbidity of HF.15 As noted, other therapies for HF, such as telemedicine interventions, appear less effective for patients with HF who have depression than for patients with HF who do not, resulting in some trials excluding patients with major depression.10,17
The prime focus on exercise is often at the expense of other important aspects of HF care. For example, in people with HF, depression and anxiety are common and are associated with adverse outcomes such as reduced adherence to treatment, poor function, increased hospitalisations and healthcare usage, and elevated mortality.18–21 However, despite the adverse impact of anxiety and depression, they are underdiagnosed and undertreated in people with HF.18 Anxiety and depression are not only common comorbidities in HF, but they are among the leading causes of burden worldwide.22 Although a variety of psychological interventions have shown beneficial effects on anxiety and depression and quality of life in people with CHD, for people with HF and depression psychotherapy appears to be effective, although more flexible depression care management approaches show promise, depending on individual needs.23,24 Other approaches such as telephone-delivered blended collaborative care have been shown to improve mental health-related quality of life and mood.25 However, despite this, some caution is urged until more evidence is available regarding the efficacy of such interventions.26
Other Important Comorbidities
It is common for people with HF to have comorbidities, such as diabetes, chronic obstructive pulmonary disease, hypertension, kidney disease, anaemia and AF, the burden of which increases with age.27 However, given that many people with HF are old, frail, with multiple comorbidities such as depression and cognitive impairment, and limited or no social support, there is a need for more holistic approaches to care and rehabilitation that address these issues, which are important to people and their families but which have often been regarded as of less importance by healthcare professionals. Frailty, for example, is common and is associated with a worse quality of life and poor prognosis.28 Therefore, a comprehensive, multidimensional assessment is needed to guide the management of this comorbidity in people with HF.29
Rehabilitation
Rehabilitation programmes for HF have traditionally been hospital based, supervised and focused on exercise as the intervention, and with the primary outcome being exercise related, such as exercise tolerance. However, it is now recognised that although exercise is central to the process and benefit of rehabilitation, other components are also essential.30 These include education, diet and nutrition counselling, smoking cessation, and psychosocial assessment and interventions when appropriate.30
Novel home-based HF rehabilitation programmes have emerged such as REACH-HF (Rehabilitation EnAblement in Chronic Heart Failure), a health professional-facilitated, self-management programme for people with HF and their carers.31 The REACH-HF intervention, delivered at the patient’s home via a mixture of face-to-face and telephone contacts over 12 weeks, includes four core elements: a manual for patients with a choice of two structured exercise programmes; a progress tracker (an interactive booklet designed to facilitate learning from experience to record symptoms, physical activity and other actions related to self-care); a family and friends resource manual for use by carers to increase their understanding of HF and carer physical and mental wellbeing; and facilitation by cardiac nurses or physiotherapists trained in using person-centred counselling and how to tailor the intervention.
This more holistic programme is yielding promising results such as improved HF health-related quality of life at 1 year and improved confidence of self-management in carers of people with HF.32,33 These promising findings support those of a review indicating that home-based CR and hybrid (centre- and home-based) CR improved functional capacity, although only the former improved health-related quality of life over usual care.34
Digital Technologies
In this era of digital health, a plethora of technologies exist, and are developing at a rapid pace, for use in rehabilitation for people with HF. Current examples include smartphones and fitness trackers to provide instruction and monitoring of exercise, with data being shared with healthcare professionals. These can help with physical activity tracking and fall prevention and are particularly useful in older patients. Other digital technologies that may be helpful for tracking exercise activity and effort include implantable device telemetry or oscillometric monitoring. App-based approaches can be used to provide education and set goals and activity schedules. Smartwatches can track heart rate, activity, oxygen saturation and ECG data. Sensors can be used to monitor people in their home, thus reassuring them that they can complete activities safely. Artificial intelligence is likely to play an increasingly important role in rehabilitation and to enhance its provision and uptake.35
Telehealth has the potential to contribute to a personalised approach and improve access to HF healthcare and overcome geographic inequalities. It can also improve HF self-management and empowerment and lead to greater efficiencies in the healthcare system.36 Teleconsultation, telemonitoring and the use of wearable devices and apps for HF health and lifestyle support are increasing rapidly, but caution is warranted regarding such technology, especially concerning issues such as data validity and privacy. Support for patients and clinicians using these technologies is an important consideration.37
Cardiac tele-rehabilitation is delivered by a range of technologies and has a similar effectiveness to centre-based programmes and may be particularly suitable for rural, remote and hard-to-reach populations.38–40 However, more evidence is needed to confirm that a telemedicine intervention confers additional health benefits for patients in the later phase of CR.41 Also, caution is urged when implementing telehealth-based interventions, given that the mean age in most studies is less than 60 years. Some patients, particularly the elderly and frail, may find such interventions challenging without facilitated support.40,42
New models of rehabilitation need to enhance reach and impact. Efforts to augment access, uptake and completion include ensuring that all people with HF are automatically referred or enrolled into rehabilitation; offering programmes that prioritise their needs, preferences and circumstances rather than those of the healthcare system; the inclusion of partners or carers; providing feedback; ensuring continuity and integration with other parts of the health and social care system, including primary care; and taking into consideration key factors associated with health education and behaviour change such as culture, ethnicity, language and health literacy.43
Many of the outcomes used in rehabilitation for people with HF are determined by cardiologists and other healthcare professionals, with little input sought from patients and partners or consideration of outcomes that might be more important to them. In addition, patient and carer expectations, experience and satisfaction would bolster outcome measurement.43
Gaps in Knowledge
There remain gaps in our knowledge, such as a lack of definitive evidence of the effectiveness of exercise rehabilitation in people with HF with preserved ejection fraction. Unlike a drug, rehabilitation is a complex intervention: it does not have a standard dose, formulation or frequency and it cannot be double-blinded in trials. It is varied and depends largely on patient choice, motivation and adherence, but also on a host of other contextual factors such as the need for it, the setting in which it is delivered, the knowledge, skills, competencies and values of the person(s) delivering it, and the resources and support available.44 The mechanisms by which such interventions work, for whom, when and why are unclear, and more nuanced approaches are needed for this to be elucidated.45
More specific knowledge gaps include the absence in most studies of HF rehabilitation of patients with end-stage HF and left ventricular assist devices (LVADs). This is likely to be due to the severe exercise limitations of patients with LVADs, although these devices can improve survival, quality of life and functional capacity.46,47 But studies to explore the possible ways of providing CR for these patients are warranted.
It would also be of interest to determine which protocols and settings improve patient adherence: for example whether, in a particular setting, there are differences in effectiveness between interval and resistance training protocols.
Why Aren’t More People with HF Offered Cardiac Rehabilitation?
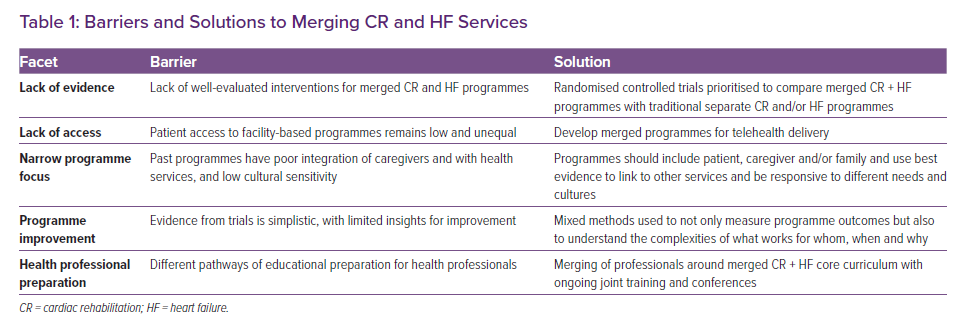
Given the evidence of the possible benefits of CR for people with HF, it is puzzling as to why more HF patients are not routinely offered CR or access to CR services. This curious lack of integration reflects far less the relatively similar care needs and care pathways between patient groups than a deeper and historical bifurcation between the sub-disciplines in the cardiology of HF (and its disease management) and lifestyle–behavioural focused CR. Practitioners and researchers have discussed, researched, and practised HF and CR as if these were separate unconnected endeavours. For example, randomised trials of CR have often excluded people with HF, while HF disease management interventions were developed and evaluated, in the vast majority, as discrete ‘disease’-specific interventions. Similarly, cultures of ‘research impact’ and careerism tend to incentivise the over-specialisation of new but increasingly niche new knowledge, giving rise to researchers who prioritise personal career advantage over patient need.48 With an increasing focus on fiscal realities and health system precarity, it is not credible to maintain such questionable divisions between HF and CR patients and models of care (Table 1).
Changes to practice could also come from better and more specific research that better responds to the complexity of different HF patient populations and CR interventions. To promote knowledge development and better integration of HF patients into CR services, the narrow and simplistic nature of past attempts to evaluate cardiac services must be challenged.43,44 As an alternative to past approaches that used trials extensively to evaluate whether and how much patients with HF benefit from integration with CR services (i.e. whether CR works for HF patients), multi-method research is needed to address more nuanced questions of what works for whom, when, and why.49 This could be achieved by combining traditional trial methods to measure and evaluate outcomes, with qualitative and other mixed methods, to understand different outcomes by patient groups. This will generate new knowledge of which HF patient groups are most and least likely to benefit from variations in CR services. This more complex evaluation is congruent with trial methodology but can better identify the optimal size, scope, and delivery method of CR for different HF population groups, such as patients with more symptomatic HF (New York Heart Association class III) or patients with multiple intersecting sociodemographic factors, such as black women.50
In addition to identifying benefits across different patient groups, research should also extend from merely measuring service outcomes to understanding these outcomes: that is, the mechanisms of intervention effects and how these vary by population and context.45,51 This could provide valuable insights into how CR works (or doesn’t), and enable services to be better optimised over time for different patient groups. Past systematic reviews of the mechanisms of HF disease management interventions have highlighted the considerable hidden complexity of HF disease management programme mechanisms, with the effectiveness of treatments for these patients being highly dependent on their prior understanding of HF and its self-care, and the ability of programmes to involve family caregivers and address underlying issues related to psychosocial wellbeing.52 These insights can then be used to improve programmes.
The imperative of viewing and handling the interventions as complex interventions in this manner is now well justified and explained in multiple iterations of the Medical Research Council’s frameworks for the development and evaluation of complex interventions and in realist methodology.4,49,53,54
A Way Forward
It is timely to consider more creative, flexible, patient-responsive and cost-effective approaches to rehabilitation for people with HF (supported by telemedicine) that are easily accessible (at home, in the community or in the hospital). Home-based approaches are an attractive option, often in conjunction with telehealth, and compare favourably with centre-based ones in terms of hospitalisation, health-related quality of life and cost.40 Telehealth-based interventions, although promising and appealing, might pose challenges to some people, especially those who are old, frail or cognitively impaired. Whatever approach is adopted the core elements should, depending on an assessment of individual need and choice, consist of education, lifestyle change, exercise and psychosocial support, unless contraindicated due to medical reasons (Table 2).55 Provision should be tailored according to individual circumstances, such as with regard to age and frailty, and possibly for recipients of cardiac implantable electronic devices or LVADs.
Conclusion
The global burden of HF and the demand for rehabilitation reinforces the need for expansion of evidence-based, home-based, digital-supported and community-based rehabilitation models of provision and a shift away from the traditional model of centre-based CR.56 For people with HF, exercise remains a central element, but so too should education, lifestyle modification and psychosocial support. More creative, flexible and individualised, needs-led approaches, exploiting the latest digital technologies, may help fill the existing gaps, improve access, uptake and completion and ensure optimal health and wellbeing for people with HF and their families.