Data from the National Health and Nutrition Examination Survey (NHANES) 2013–2016 suggests an estimated 6.2 million people in the US over 20 years old have heart failure (HF), an increase from 5.7 million in 2009–2012.1 With an annual incidence of about 1 million, the number of people affected in the US is expected to grow to more than 8 million by 2030.1,2 The financial burden is monumental; in a given year, 809,000 hospital discharges, 2 million primary care visits and 414,000 emergency department (ED) visits are due to a primary diagnosis of HF.1 This leads to an annual cost of US$30.7 billion as of 2012, with a projected cost of US$69.8 billion by 2030.2 Furthermore, patients with HF suffer from high rates of adverse clinical outcomes. HF carries a 50% 5-year mortality rate and median survival is 5–6 times less for people with HF compared with the general US population.3,4 Given the financial, medical and public health burden, HF is understandably a target for numerous established and novel interventions.
With multiple pharmaceuticals shown to benefit cardiovascular outcomes in HF with reduced ejection fraction (HFrEF), support for the initiation of comprehensive disease-modifying medical therapy (CDMMT) – including an angiotensin receptor-neprilysin inhibitor (ARNI), evidence-based β-blocker, mineralocorticoid receptor antagonist (MRA) and a sodium-glucose cotransporter 2 inhibitor (SGLT2i) – has come to the forefront of HFrEF care.5 These four pillars of HFrEF therapy are known to reduce all-cause mortality and morbidity in a cost-effective manner; however, they are underused worldwide.
The purpose of this review is to discuss the current gap in the use of CDMMT, before discussing the benefits of the newest inclusions to guideline-directed medical therapy (GDMT), including SGLT2is and ARNIs. It will cover the efficacy, value, tolerability and safety of these new therapies and will end with suggestions for the initiation and uptitration of CDMMT with potential pathways to guide treatment.
Use of Guideline-directed Medical Therapy: The Gap to Fill
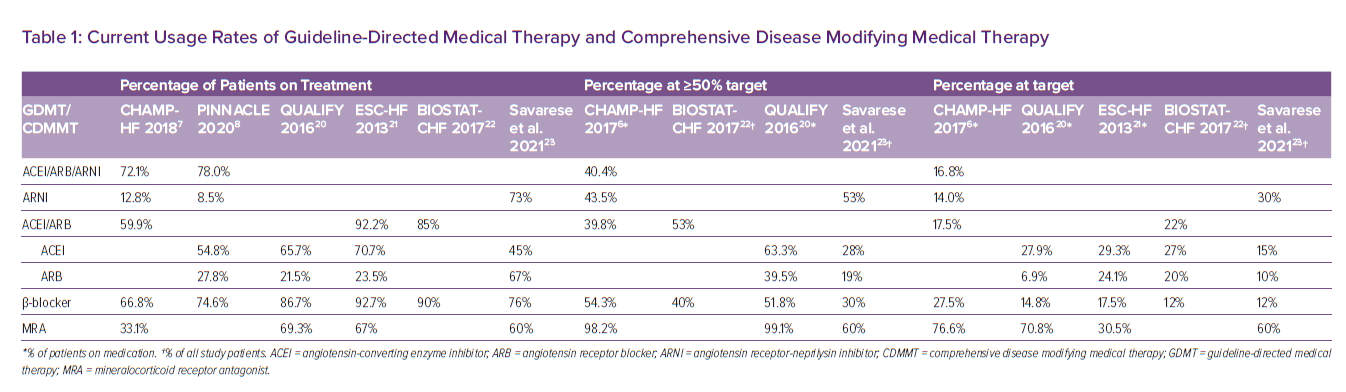
Despite the abundance of data supporting the benefits of GDMT and CDMMT, its use in the US is inadequate. The CHAMP-HF registry includes 5,000 outpatients with HFrEF on at least one GDMT medication. It encompasses data from more than 150 cardiology practices across the country. Data was collected for 2 years or until patient withdrawal or death. Analysis from 2018 showed that one-third of eligible patients with HFrEF were not prescribed an angiotensin-converting enzyme inhibitor (ACEI), angiotensin receptor blocker (ARB), or ARNI; one-third were not prescribed a β-blocker; and two-thirds were not prescribed an MRA. ARNIs have been shown to be clinically superior to ACEIs yet are still being underused and 86% of patients without a contraindication to ARNI initiation were not being treated.6,7
Similar data is available from the US PINNACLE registry, the largest outpatient cardiovascular practice registry to date, including over 6 million patients cared for by 8,800 providers. As of 2017, more than 700,000 HFrEF patients were included in the registry. Rates of use from PINNACLE were slightly better than CHAMP-HF, suggesting 74.6% of HFrEF patients were at least receiving a β-blocker; 78% were at least receiving an ACEI/ARB/ARNI; and 72.8% were receiving both a β-blocker and an ACEI/ARB/ARNI. However, the use of ARNIs is lacking, with only 8.5% on treatment.8
SGLT2is have been known to reduce major adverse cardiovascular events in people with diabetes; however, in 2020, the FDA approved the SGLT2i dapagliflozin for the treatment of all-comers with HFrEF given its reduction in worsening HF or cardiovascular death.9–11 This was followed by the formal recommendation of SGLT2is in both the 2021 European Society of Cardiology (ESC) Guidelines for Heart Failure as well as the 2021 updates to the 2017 American College of Cardiology (ACC) Expert Consensus Decision Pathway on HFrEF treatment.12,13 Although shifts in prescription patterns are expected, the most recent data suggest current uptake is low; among people with diabetes in CHAMP-HF, only 2% were being treated with SGLT2i; in contrast, people with diabetes had similar baseline rates of ACEI/ARB/ARNI, β-blocker, and MRA use, compared to people without diabetes.14 Again, the CHAMP-HF database ran from 2015 to 2017; more contemporary studies will clarify whether its use has changed now that SGLT2is have been formally recommended as a treatment for HFrEF.
Taken together, data from CHAMP-HF and PINNACLE suggest a massive therapeutic gap in the US, with up to one-third of patients not on individual components of GDMT. Worse still, the use of more novel therapies like ARNIs is lacking, and suggests a need to move away from the prior mainstays of ACEIs and ARBs. Available data for the use of SGLT2i are similarly poor, but monitoring is worthwhile given the medication was only recently recommended for the treatment of HFrEF.
Target Dosing and Titration of GDMT/CDMMT Over Time
It is well known that medications like ACEI/ARBs and β-blockers not only improve cardiovascular outcomes in HFrEF patients, but that higher doses lead to superior clinical results.15–17 In the US, the use of optimal target dosing for HFrEF therapy is poor. Using 2015–2017 data derived from CHAMP-HF, among those on ACEIs or ARBs, only 18% of patients were at target; similarly, 14% of ARNI users and 28% of β-blocker users were at target. Out of all patients included in the study (n=3,158), only 37 (1%) were prescribed the target dose for all ACEI/ARB/ARNI, β-blocker and MRA.7
Clearly titration to target dosing is an issue. The IMPROVE-HF study evaluated the effectiveness of a quality improvement intervention for the use of GDMT. It included 167 outpatient cardiology practices with more than 34,000 patients and was completed in 2009. Rates of target dosing only increased modestly over the 2-year follow up period, with ACEI/ARB increasing from 36.1% to 37.9%, β-blocker increasing from 20.5% to 30.3%, and MRA increasing from 74.4% to 78.4%.18 More recent data on 2,500 outpatients from the CHAMP-HF registry suggests use and target dosing has not improved since. In CHAMP-HF, patients were followed for medication titration over time. At 12 months follow-up, the proportion of patients who had GDMT initiated or increased at 12 months was 7% for ACEI/ARB, 10% for ARNI, 10% for β-blocker, and 6% for MRA. In contrast, those who had discontinued GDMT or had decreased dosing were 11%, 3%, 7%, and 4%, respectively. Less than 1% of all patients were treated with target doses of ACEI/ARB/ARNI, β-blocker and MRA.19 Findings thus suggest target dosing is extremely low despite sufficient time for uptitration and it is clear that optimising CDMMT and GDMT to therapeutic doses needs to be addressed at a national level.
International Use and Dosing of GDMT
Internationally, the data appears to be slightly better than the US. Performed between 2013 and 2014 in more than 36 countries around the world, the QUALIFY registry is an observational, longitudinal, prospective survey of over 7,000 HF patients who were recruited after hospitalisation for acute decompensated HF. GDMT usage was higher than CHAMP-HF and PINNACLE, with 65.7% of patients on ACEIs, 86.7% on β-blockers and 69.3% on MRAs.20 Similar numbers are noted in the ESC-HF Long-Term Registry, which ran from 2011 to 2013 and included 12,440 patients from 21 European countries. The registry incorporated data from inpatients with acute decompensated HF and outpatients with chronic HF. At time of discharge, those who were hospitalised had 77% ACEI/ARB usage, 71.8% β-blocker usage and 55.3% MRA usage; the rate of GDMT usage significantly increased compared to their pre-hospitalisation values, suggesting initiation of GDMT during an inpatient stay. Outpatients with HFrEF had even higher usage rates, with 92.2% ACEI/ARB usage; 92.7% β-blocker usage; and 67% MRA usage.21 Overall, data from both QUALIFY and the ESC-HF Registry seems to suggest that the use of GDMT is somewhat higher outside the US (Table 1).
However, the proportion of patients at target dose was comparably low. In the ESC-HF Long-Term Registry, target dosage rates were 29.3% for ACEI users, 24.1% for ARB users, 17.5% for β-blocker users and 30.5% for MRA users.21 In the QUALIFY registry, among individuals on medication, those at ≥50% target dose and 100% target dose was 63.3% and 27.9% for ACEIs; 39.5% and 6.9% for ARBs; 51.8% and 14.8% for β-blockers; and 99.1% and 70.8% for MRAs, respectively.20 Similar findings have been noted in BIOSTAT-CHF, a registry that included 11 European countries with 2,100 HF patients. When it was published in 2017, among all study participants, those at ≥50% target dose and 100% target dose was 53% and 22% for ACEI/ARBs, and 40% and 12% for β-blockers, respectively.22 Overall, the use of certain therapies appears better than in the US, but optimal utilisation is equivocally lacking.
The data presented in the aforementioned studies are derived from registries; real-world data are similarly dismal. A recent multinational study analysing healthcare databases from the US, UK and Sweden cements the findings of suboptimal titration, as well as high rates of premature discontinuation.23 In patients who have been hospitalised with a recent diagnosis of HF and subsequently initiated on GDMT, after a follow-up of 12 months, target dosage rates were 15% for ACEIs, 10% for ARBs, 12% for β-blockers and 30% for ARNIs. MRAs, in contrast, reached target dose at a rate of 60%. Discontinuation rates were far higher than CHAMP-HF, reaching 55% for ACEIs, 33% for ARBs, 24% for β-blockers, 27% for ARNIs and 40% for MRAs.19,23
Should We Fill the Gap? The Additive Benefit and Impact of Optimal Treatment
The effects of such a lapse in treatment are profound. Numerous studies have shown an incremental benefit of each component of GDMT when added to background HF therapy. As an example, the addition of β-blocker to ACEI/ARB is associated with higher 2-year survival rates for HFrEF patients.24 Furthermore, analysis of the QUALIFY registry noted that at 18 months, adherence to GDMT recommendations was associated with a reduction in death due to HF as well as the composite of cardiovascular death or hospitalisation for HF.25 Failing to treat, unsurprisingly, is associated with the opposite; in BIOSTAT-CHF, reaching <50% of target dose was associated with worse survival.22 Similar concerns regarding morbidity of HF were noted in a subsequent study from the PINNACLE registry, which looked at 11,000 patients with stable HFrEF. As may be expected, the majority of those with an acute decompensation were undertreated, with 42.4% on one medication and 43.4% on two medications. Worse still, 40–50% of patients were on suboptimal dosing, defined as less than 50% of the target dose. Given that the mean time to event was 1.5 years after the initial diagnosis of HFrEF, there was ample time for uptitration of therapy, yet it did not occur.26
Transitioning from the old mainstays of GDMT to the novel regimen of CDMMT is similarly important for patient outcomes. A cross-trial analysis of EMPHASIS-HF, PARADIGM-HF and DAPA-HF sought to evaluate the benefit of CDMMT (ARNI, β-blocker, MRA, and SGLT2i) compared to conventional therapy (ACEI/ARB and β-blocker). When compared to conventional therapy, CDMMT would be expected to lower the risk of cardiovascular death or hospital admission for HF by over 60% (HR 0.38; 95% CI [0.30–0.47]). Similarly, CDMMT would be expected to reduce the risk of all-cause mortality by just under 50% (HR 0.53; 95% CI [0.40–0.70]). Treatment with ARNI, β-blocker, MRA and SGLT2i could add between 2.7 and 8.3 additional years free from cardiovascular death or HF hospital admission and between 1.4 and 6.3 additional years of survival.27
While there is a clear benefit to shifting from GDMT to CDMMT, the lack of use comes at a cost. Older studies have suggested that in the US, optimal implementation of ACEI/ARBs could save over 6,000 lives annually; β-blockers over 12,000 annually; and MRAs over 20,000 annually. When accounting for all GDMT therapies, almost 68,000 lives could be saved.28 Optimal use of more novel therapeutics, namely ARNIs, could potentially prevent another 28,000 deaths annually.29 One recent study used a decision analytical model to approximate the magnitude of the benefit of optimal implementation of SGLT2is for the HFrEF population in the US. Extrapolating from DAPA-HF, SGLT2is could prevent more than 34,000 deaths each year.30 In sum, every 10% improvement in guideline directed care is associated with 13% lower odds of 2-year mortality risk.31
The impact goes beyond the projected mortality rates of optimal treatment; all medications in GDMT and CDMMT are considered cost effective and have high/intermediate value, with some even considered cost-saving. The newer treatments are more expensive than the old GDMT mainstays, yet the incremental cost-effectiveness ratio (ICER) of dapagliflozin, based on DAPA-HF outcomes, is US$8,000–11,000 per quality-adjusted life year (QALY).32 In addition, the ICER of an ARNI (compared to an ACEI) is US$23,000–45,000 per QALY.33–35 Both of these novel therapies fall under the high value category (ICER <US$50,000) as stated in the 2014 ACC/AHA Statement on Cost/Value.36 Even better, β-blocker and MRA are considered cost dominant, meaning they are both clinically superior and cost-saving.37 Incremental cost-effectiveness analysis for GDMT, namely ACEI/ARB, β-blocker and MRA, have noted cost-effectiveness, as well as cost-savings for each medication added to a patient’s regimen. Specifically, the ICER for ACEI + β-blocker compared to ACEI alone, as well as the ICER for ACEI + β-blocker + MRA compared to ACEI + β-blocker was <US$1,500 per QALY.38
Thus, the traditional treatment of HFrEF with GDMT, as well as the more novel approach with CDMMT, have dramatic cost/benefit ratios and could potentially save thousands of lives (and dollars) annually. Despite this, barriers to treatment exist, including gaps in knowledge and awareness of CDMMT, therapeutic inertia, concerns about drug safety and side-effects and uncertainty surrounding the effectiveness of treatment.39 Use in the US is uniquely hindered by large variability in pharmaceutical pricing, as well as high out-of-pocket costs and the need for prior authorisations for the more novel ARNI and SGLT2i.12,40–42 A call for reform of the utilisation management requirements and prior authorisation process signed by 17 medical organisations, including the ACC and the AMA, hopes to curb the negative impact felt by patients.43 Whether this will improve timely and affordable access to optimal care remains to be seen.
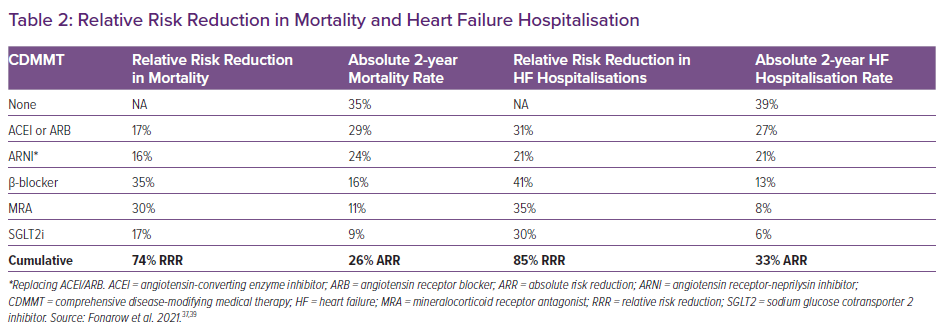
The Push for Early Initiation – Rationale and Safety
CDMMT is presumed to reduce the risk of death by 74% over a 2-year period, leading to a number needed to treat of just four (Table 2); thus timely initiation is paramount to the treatment of HFrEF.37 Such a benefit is quick to occur. With regard to the mainstays of GDMT, initiation of carvedilol against a background of ACEI/ARB in the COPERNICUS trial suggested benefit for both all-cause mortality and for the combined endpoints of death, hospitalisation or withdrawal as early as 14–21 days after initiation of treatment.44 Findings for metoprolol succinate in the MERIT-HF trial were concurrent, with the reduction in all-cause mortality/all-cause hospitalisation occurring by week 8.45 Finally, EMPHASIS-HF noted a benefit with MRA in reducing the endpoint of cardiovascular mortality and HF hospitalisation as early as 30 days.46
Similar findings are noted for CDMMT. ARNIs were first studied in the stable HF population in the PARADIGM-HF trial; treatment protocol indicated that sacubitril-valsartan should be started and uptitrated within 4–6 weeks and the benefit of reducing the risk of death and hospitalisation for HF was noted soon after.47 Subjective improvement with ARNIs occurred quickly as well; in a subsequent analysis of the same trial, there was a greater mean improvement in self-reported health status based on the 12-item Kansas City Cardiomyopathy Questionnaire, which occurred at a median timepoint of 57 days.48 For SGLT2is, the EMPEROR-Reduced trial showed that empagliflozin reduced the combined risk of death, hospitalisation for HF, or emergent/urgent HF visit requiring IV treatment as early as 12 days after initiation.49,50 In subsequent analysis of DAPA-HF, dapagliflozin was shown to reduce the composite endpoint of cardiovascular death or worsening HF as early as 28 days after randomisation, with a sustained significant benefit throughout the study.11,51
Given this quick onset of medical benefits to the patient, initiation of all GDMT/CDMMT medications should be prompt; of top concern, however, is whether such a multi-drug regimen is safe. Safety of additional therapy is well demonstrated when analysing the randomised control trials that established GDMT. In the original β-blocker trials, over 95% of subjects were already on ACEI/ARBs, and for MRAs, over 90% of EMPHASIS-HF enrollees were already on ACEI/ARBs and over 85% were on β-blockers.46,52–55 For newer therapies, in PARADIGM-HF, 93% of patients were on β-blockers and 56% were on MRAs; fewer patients in the ARNI group stopped their medication for an adverse event, compared to those in the control group (enalapril).47 In DAPA-HF, 95.1% of patients were on an ACEI/ARB/ARNI, 96% were on a β-blocker, and 71.5% were on an MRA, yet frequency of adverse events did not differ between the dapagliflozin group and the control group (placebo).11 Similar baseline therapy rates were comparable in EMPEROR-Reduced, which compared empagliflozin to placebo and found with the exception of genital tract infections, there was no significant difference in adverse events.50 Taken together, the components of GDMT and CDMMT should be consider safe to use with one another.
With these safety profiles and the quick onset of benefit, the question then becomes whether such medications are safe and/or more effective when started quickly, namely in the inpatient setting or whether titration needs to be prolonged to prevent side-effects. Available studies support the former. Medications that were first shown to be safe for initiation prior to hospital discharge included GDMT, namely β-blockers (specifically carvedilol), ACEI/ARBs and MRAs.56–59 The benefit of early initiation is certainly present for β-blockers and ACEI/ARBs; in observational studies, β-blocker initiation prior to hospital discharge was associated with lower mortality and lower readmission rates.60,61 Similar findings have been noted for ACEI/ARBs started prior to hospital discharge.57,62 MRAs, in contrast, have been associated with improved overall survival in some studies and lower risk of HF rehospitalisation in others, but the findings are not as consistent.63–65 Nonetheless, the available data suggests GDMT medications should be started while individuals are in hospital prior to discharge. Fortunately, national trends suggest this is the case; in the GWTG-HF registry, 90% of treatment-naïve HF patients were initiated on β-blocker and 87% were initiated on ACEI/ARB during hospitalisation or at discharge. However, only 25% were initiated on MRA.66
As opposed to the observational studies for GDMT inpatient initiation, the more novel CDMMT are the subject of more proactive trials. PIONEER-HF evaluated ARNI initiation specifically in those with acute decompensated HF. ARNIs were not only safe in the context of acute HF, but they were also associated with a greater reduction in NT-proBNP; further, in exploratory analyses, ARNIs were associated with reduction in the composite of cardiovascular death or rehospitalisation from HF as soon as 30 days after initiation.67,68 Similar findings were noted in the safety-driven TRANSITION trial, wherein patients treated for acute decompensated HF were randomised to ARNI initiation either prior to hospital discharge or within 14 days of discharge; safety endpoints were similar for both strategies, indicating no significant disadvantage to early initiation of ARNIs.69
With the remarkable findings of rapid benefit in EMPEROR-Reduced and DAPA-HF, the SOLOIST-WHF trial was specifically designed to show that an SGLT2i could safely be started before or shortly after hospital discharge for acute decompensated HF; sotagliflozin was initiated prior to discharge in 48.8% of patients or at a median of 2 days after discharge in 51.2%. Compared to placebo, sotagliflozin reduced the primary endpoint of cardiovascular death and hospitalisations/urgent visits for HF and with the exception of diarrhoea and severe hypoglycaemia, safety endpoints were similar between the two treatment arms.70 Two ongoing trials, EMPULSE and DAPA ACT HF-TIMI 68 (NCT04363697), are further evaluating the clinical benefit of SGLT2i in patients hospitalised with HF.71
Both GDMT mainstays and the more novel therapies of CDMMT can be used together safely. Furthermore, they can be safely initiated and uptitrated quickly, without concern for higher rates of adverse events. Given their dramatic benefit for cardiovascular outcomes, such early initiation and rapid titration of GDMT and/or CDMMT needs to occur as soon as a diagnosis of HFrEF is made.
Simultaneous/Rapid Sequence Initiation and Optimal Titration: A Conceptual Framework and a Call for Action
A conceptual framework for the rapid initiation of CDMMT for HF is readily available, but bears repeating.5,12,13,37,72 The aforementioned observational studies in the US and around the world suggest that ARNIs are beneficial compared to ACEI/ARBs, yet they are extremely underprescribed; a reasonable step is thus to convert all HFrEF patients on ACEI/ARB to ARNI, barring any contraindication. It should be noted that there is a difference in US guidelines compared to other countries. According to the ACC, ARNI is preferred, but if ARNI administration is not feasible, then an ACEI/ARB can be offered instead; per the ESC, either ARNI or ACEI/ARB can be offered as a first-line option.12,13 β-blockers are cost-dominant and are being used at a decent rate, but target dosing could be improved. MRAs are also cost-dominant, yet despite their low cost, they are underused and frequently not titrated to target dose. Finally, SGLT2is have been shown to be a cost-effective and beneficial addition to the mainstays of HF therapy but as they were only approved for HFrEF within the past year, data on usage have not yet been described.
These four medications should be started and uptitrated in a timely manner to derive the highest benefit for the HFrEF patient. The rationale goes beyond the reduction in cardiovascular outcomes. Treatment with an ARNI, compared to an ACEI, has less risk of severe hyperkalaemia, which could reduce discontinuation of an MRA.5,73 Treatment with an SGLT2i reduces the worsening of renal function and delays progression to end-stage renal disease, which may allow for longer usage of ARNIs and MRAs.5,10 While some may feel uncomfortable with a rapid initiation of multiple medications for HFrEF, there is no evidence to date that suggests such a strategy would produce adverse events; in fact, a delay in treatment would lead to unnecessary clinical worsening and cardiovascular death.22,27,31,37
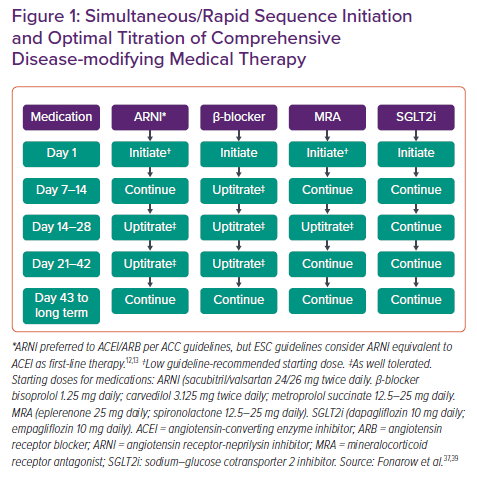
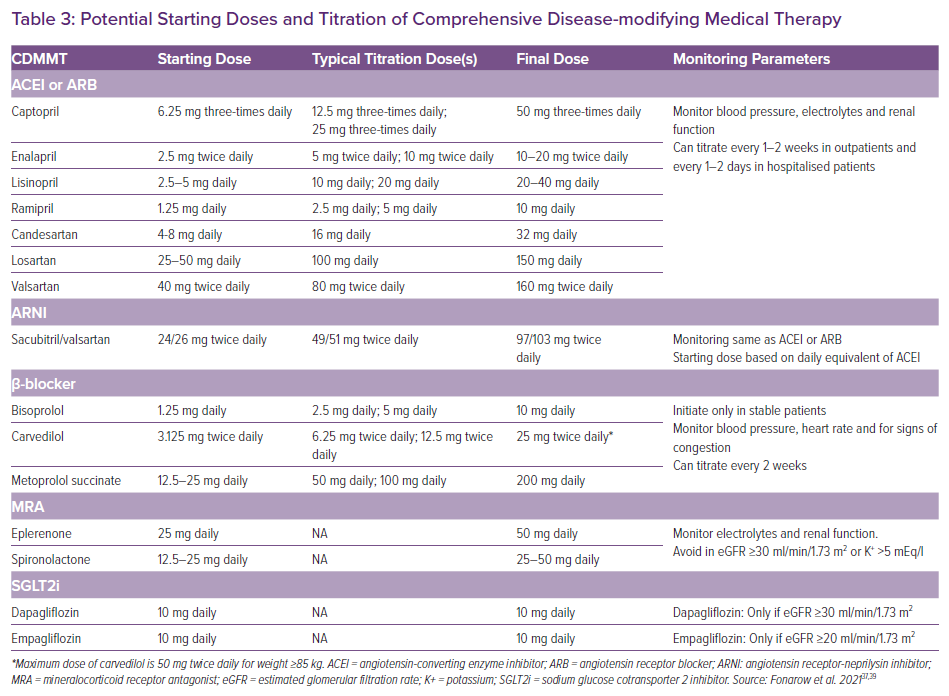
Suggestions on initiation and titration of CDMMT are shown in Table 3 and Figure 1. All four medications can be started upon diagnosis of HFrEF, including in the inpatient setting prior to discharge. Medications should be started at a low dose. At 7–14 days, β-blocker can be uptitrated; at 14–28 days, the ARNI, β-blocker and MRA can all be uptitrated; and at 21–42 days, the ARNI and β-blocker can be increased to their maximum dose. By 2 months, the patient can safely be taking the maximum dosing of CDMMT.
Throughout initiation and titration of CDMMT, the patient should have their volume status monitored with the goal of euvolaemia. If congestion is present, the patient should be initiated on a loop diuretic, which can be titrated to the relief of congestion. Though they lack the benefits to mortality of CDMMT, diuretics alleviate HF symptoms and reduce HF hospitalisations. Providers should be aware that diuretic dosing can change in the setting of increased CDMMT dosing and may even be reduced or stopped altogether. Only once maximal dosing of CDMMT is established should additional HFrEF therapies be considered. Such medications include hydralazine/isosorbide dinitrate for persistent symptoms in black patients, ivabradine for patients with a resting heart rate above 70 BPM, and vericiguat for all patients with persistent symptoms.12,13
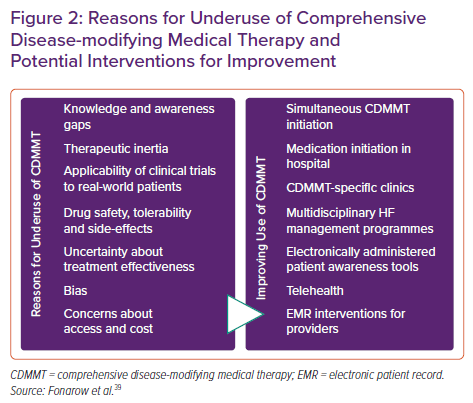
Evidence-based mechanisms to facilitate ongoing CDMMT usage and titration are numerous and should be used to ensure maximum benefit. Such strategies include enhancing patient awareness through electronically-administered activation tools, improving provider awareness through the electronic medical record and employing both in-person and telehealth GDMT clinics designed for initiation and titration of medications (Figure 2).39,74–79
Conclusion
Despite an abundance of evidence for the benefit of HFrEF medical therapy, data from the US and around the world suggests that the use of GDMT and CDMMT has substantial treatment and dosing gaps. Both the use of medications, as well as increasing medications to optimal dosing, needs substantial improvement to derive the maximum benefit of HFrEF treatment. The mainstays of therapy, the four pillars of CDMMT, are proven to be safe, effective and well tolerated. These therapies can be started at the time of HFrEF diagnosis, including in-hospital, at a low dose and then optimally titrated over time. By following a simple and effective algorithm for the initiation of CDMMT, the quality of HF care can be improved with the potential for tens of thousands of lives being saved.