Heart failure (HF) is a major global non-communicable health problem that is estimated to affect at least 64.3 million people worldwide, with the prevalence of known (clinically diagnosed) HF in the general adult population being 1–2%.1,2 In fact, following normal childbirth, HF is the most common reason for hospitalisation.3 Moreover, approximately 2% of the total annual healthcare budgets in Europe and the US is spent on HF-related care.4 HF is also deadlier than some of the common malignancies in the general population, such as prostate and bladder cancer in men and breast cancer in women.5 Although the incidence of HF remains fairly constant, large population studies have reported an increase in the prevalence of HF over time.6 This is important because the observed increase will inevitably lead to further increases in hospitalisation rates, with a concomitant increase in health care expenditures. Hence, there is a need for a cost-effective prognostic biomarker in HF beyond natriuretic peptides. In light of available evidence, the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) consensus meeting recommended a multimarker approach, including cardiac troponins, natriuretic peptides and soluble suppression of tumorigenicity-2 (sST2).7
In this review, we focus on the role of carbohydrate antigen (CA) 125, a high molecular weight transmembrane glycoprotein most commonly associated with ovarian cancer.8 CA125 is normally expressed on the cell surface in various tissues, including the pleura, pericardium, peritoneum, endometrium, endocervix, salpinges, lung, conjunctiva and prostate.9 The physiological role of CA125 is to hydrate and lubricate epithelial luminal surfaces, which protects them against mechanical stress and stretch imposed on the cells.8 Furthermore, the interaction between transmembrane mucins and adjacent proteins supports the role of CA125 in processes involving fluid and cell transport, inflammation, tissue repair and tumour dissemination.10,11 Finally, CA125 has been shown to modulate both innate and adaptive immune processes, such as suppression of natural killer (NK) cell activity and regulation of galectin activity.12,13
Increased plasma CA125 concentrations are not exclusive indicators of neoplastic states, because CA125 levels are normally elevated during menstruation and in the early stages of pregnancy.14 Furthermore, CA125 is upregulated in multiple pathological states, such as liver cirrhosis, pelvic inflammatory disease, peritoneal trauma, ascites and lung malignancies.15 However, the clinical use of this biomarker has been predominantly associated with the work-up of patients with suspected or diagnosed ovarian cancer.16 In fact, CA125 has served as the main biomarker for ovarian cancer for almost four decades, playing an important role in the treatment and follow-up phases of ovarian cancer management.16,17
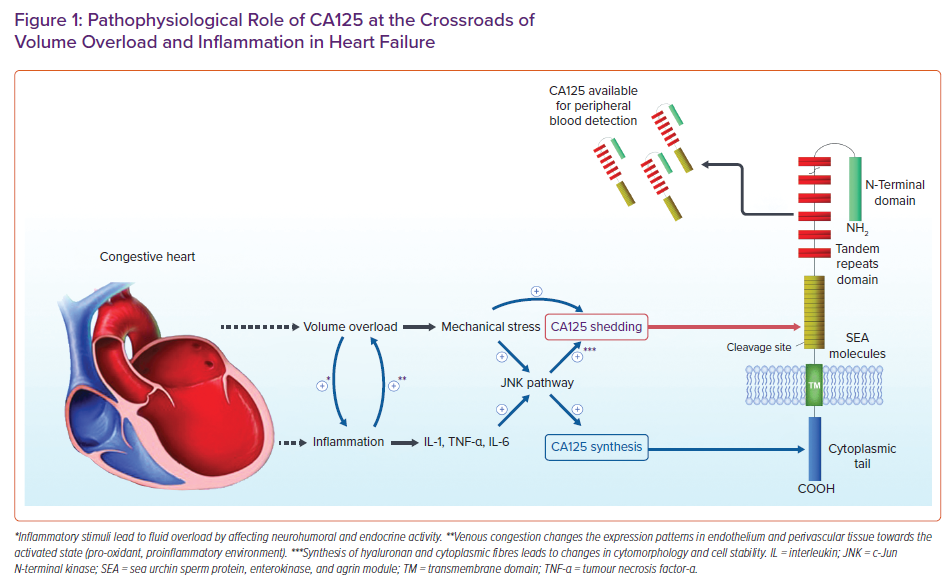
Apart from being elevated in many physiological and pathological conditions, accumulating evidence implicates CA125 in pathophysiological processes underlying HF.18,19 Thus, in this review, we present the molecular background regarding the role of CA125 in HF and address valuable clinical aspects concerning the relationship between CA125 and both prognosis and the therapeutic management of HF.
Challenges Assessing Congestion in Heart Failure: Urgent Need for Improvement
The development of congestion leading to HF decompensation is a strong predictor of poor patient outcomes.20 Thus, the timely detection of congestion, and subsequent monitoring, is vital before it leads to cardiac decompensation. However, accurate quantification of congestion in daily clinical practice is challenging. Quantification is very challenging when the extrapulmonary signs of congestion are mild, such as in the setting of acute pulmonary congestion due to hypertension or in patients near discharge after HF hospitalisation. Although increased intracardiac filling pressures can frequently precede the appearance of overt congestive symptoms by days or weeks, the increase is often subtle and difficult to detect, and may be masked by other comorbidities.21
Clinical scoring systems combining several clinical variables have been shown to assess the level of congestion more accurately than any individual indicator used in isolation. Among the many scoring systems developed, evidence is strongest for the EVEREST score, derived from the EVEREST trial, and it appears to be the best candidate for routine use in AHF management.20,22 Another valuable tool to assess congestion is lung ultrasound (LUS), which enables precise assessment of extravascular lung water.23 Aside from the CHAMPION trial, there is scarce evidence that HF management guided by standardised congestion assessment strategies results in a better prognosis.21 Thus, invasive strategies, such as pulmonary artery pressure-guided HF management should only be considered in a select group of patients in whom mechanism of clinical deterioration is unclear. Importantly, natriuretic peptide-guided therapy in high-risk patients with HF yielded disappointing results with regard to clinical outcomes.24
Interaction Between CA125 and Congestion and Inflammation in Heart Failure
The exact mechanisms that could explain elevated plasma CA125 concentrations in the setting of HF are yet to be fully elucidated. However, accumulating data suggest that the observed increase in circulating CA125 concentrations in HF is due to at least two pathophysiological mechanisms that partially overlap.25 It has so far been well established in a various malignant and non-malignant pathologies that CA125 correlates with physical and objective signs indicative of fluid congestion and effusions. For example, serum CA125 concentrations are positively correlated with pleural effusion volume in patients with chronic obstructive pulmonary disease, as well as with serosal fluid accumulation and ascites in patients with ovarian cancer and other non-ovarian benign and malignant diseases.26,27 Accordingly, Núñez et al. reported that the presence of clinical signs associated with volume overload was most robustly related to increased serum CA125 concentrations in acute HF.28 Even in the setting of ovarian cancer, CA125 concentrations were shown to be more significantly associated with ascites volume and peritoneal carcinomatosis than with the ovarian mass volumes and carcinoma per se.29
The mediator between volume overload and elevated CA125 concentrations is the mechanical stress produced by excessive fluid accumulation. Subsequently, increased mechanical stress or inflammatory stimuli (as discussed below) trigger c-Jun N-terminal kinase (JNK) pathways, resulting in two cellular changes that lead to an increase in CA125.30 First, activation of JNK stimulates the synthesis of CA125. Second, because CA125 is linked to the actin cytoskeleton, the change in the morphology the stability of the cell membrane, along with the mechanical stress, activates the O-glycosylated extracellular domain of CA125, shedding it from mesothelial cells and thus increasing its concentration in the periphery.31
Nevertheless, CA125 seems to be associated with increased 6-month mortality independent of evidence of fluid overload, indicating the involvement of CA125 in other pathogenic processes underlying HF.25 Inflammation has arisen as a viable culprit, because correlation between CA125 and proinflammatory cytokines, such as tumour necrosis factor (TNF)-α, interleukin (IL)-6 and IL-10, has been identified.32 In addition, Zeillemaker et al. demonstrated that CA125 secretion could be enhanced by the inflammatory cytokines IL-1β, TNF-α and lipopolysaccharide of Escherichia coli.33 As noted above, the proposed mechanism by which systemic inflammation affects CA125 concentrations also involves JNK molecular pathways. Notably, it has been established that venous congestion changes expression patterns in the endothelium and perivascular congested tissue towards the activated state, leading to upregulation of pro-oxidant, proinflammatory and vasoconstricting factors.34 Accumulating data suggests that CA125 may even play a role in the cardiac remodelling process by regulating galectin activity or modifying the mass and stiffness of the intercellular matrix. For example, in patients admitted to hospital with acute decompensated HF, positive correlations between galectin-3 and proxies of inflammation were observed only in patients with CA125 concentrations above the median value.12
Furthermore, in oedematous patients, such as those with HF decompensation, bacterial endotoxin translocation occurs from the gut into the circulation, stimulating the activation of the immune response.35 Conversely, inflammatory stimuli worsen fluid overload by affecting neurohumoral and endocrine activity.36 Overall, volume overload and inflammation in HF mutually interact, augmenting each other’s activity in a bidirectional manner, thus creating a positive feedback loop that leads to elevated CA125 concentrations (Figure 1).
CA125 as an Indicator of Congestion in Heart Failure and its Relationship With Haemodynamic and Echocardiographic Parameters
Although congestion plays an important role in the pathogenesis of acute HF, its severity and organ distribution are largely heterogeneous.37,38 Of note, fluid retention and congestion are the most common reasons for the hospitalisation of patients with worsening of HF and present important therapeutic targets in routine clinical practice.39 However, complete and efficacious decongestion in patients with HF is challenging, because residual congestion, such as that present in tissues, may be underappreciated in a sizeable number of HF patients at discharge, thus exposing them to an increased risk of early rehospitalisation and death.40 Moreover, methods for identifying and quantifying systemic congestion in clinical practice are fairly limited because two types of congestion, both implicated in the outcomes of HF patients, must be evaluated: intravascular and tissue congestion. Residual congestion at discharge strongly portends worse outcomes, whereas diuretic resistance and poor diuretic response complicate the accomplishment of euvolaemia.41
Although current expert recommendations suggest an integrative multiparameter-based evaluation of congestion, there is growing interest in the establishment of a cost-effective and reliable biomarker of fluid overload in HF.37,38,42 CA125 emerged as a potential candidate providing additional information beyond intravascular volume status, because plasma CA125 concentrations seem to be positively associated with the signs and symptoms of congestion, including peripheral oedema and serosal effusion, in patients with HF.43,44 Similarly, in patients with ST-elevation MI (STEMI) complicated with HF (Killip Class ≥II), circulating CA125 concentrations were correlated with pulmonary congestion and had similar prognostic power for mortality as high-sensitivity C-reactive protein and N-terminal pro B-type natriuretic peptide (NT-proBNP) in the STEMI population.45,46 In line with this, CA125 was shown to be associated with well-established laboratory proxies of clinical congestion, such as bio-adrenomedullin and NT-proBNP.47 In fact, one study showed that in clinical scenarios marked by systemic congestion and right ventricular dysfunction, CA125 may even outperform NT-proBNP in predicting mortality.48
The main differences between the two biomarkers are seen in their metabolism and in their relationship to age, as well as kidney and cardiac function. Specifically, CA125 has a significantly longer half-life than NT-proBNP (days versus minutes), and, in contrast to NT-proBNP, CA125 concentrations are not substantially modified by age and renal dysfunction or ejection fraction.49 Overall, because NT-proBNP is primarily a proxy of left ventricular myocardial stretch and because CA125 is better correlated with indices of right-sided HF, Núñez et al. suggested the complementary use of both markers to assess the degree of participation of each heart side.50 In practical terms, by accounting for the notable differences between the two biomarkers, CA125 may be more valuable in older patients with impaired renal function and predominant involvement of the right side of the heart, whereas NT-proBNP may be more valuable in euvolemic or mildly congested patients with predominant involvement of the left side of the heart.
Moreover, a recent meta-analysis by Li et al. showed that, among patients with acute HF, CA125 concentrations were significantly higher in those with than without pleural effusions.51 Nevertheless, in patients with refractory congestive HF treated with continuous peritoneal dialysis, CA125 concentrations decreased with decongestion, despite the presence of an osmotic solution in the peritoneum and concomitant peritoneal irritation induced by it.52 From these findings, doubts were raised as to whether the observed association between an increase in CA125 and serosal effusion merely represents parallel processes caused by a common pathophysiological culprit or a causal relationship between the two. In summary, future studies should explore the relationship between CA125 and other established laboratory and imaging markers of congestion, as well as investigate the role of CA125 in the identification of intravascular versus extravascular congestion.
Apart from clinical parameters of congestion, the authors of a pivotal study reported that CA125 concentrations are correlated with haemodynamic parameters, right atrial pressure and pulmonary capillary wedge pressure, further supporting the association between congestion and CA125.53 Importantly, the same study demonstrated that, during follow-up, CA125 concentrations decreased in patients after heart transplantation or clinical stabilisation of HF, but increased in patients in whom further deterioration of HF was recorded.
Several authors have reported a correlation between CA125 and echocardiographic parameters in HF. In an early report, Kouris et al. reported that serum CA125 concentrations were weakly correlated with approximated right ventricular systolic pressure and renal function, but no significant correlations were found between CA125 and any of the following echocardiographic parameters: E wave deceleration time on Doppler echocardiography, left ventricular ejection fraction (LVEF) or left ventricular end-diastolic diameter.54 Nevertheless, the authors of that study demonstrated that serum CA125 concentrations are associated with the clinical severity of chronic HF and are consistent with the symptoms and signs of fluid congestion.54 Conversely, D’Aloia et al. showed that serum CA125 concentrations were related to echocardiographic parameters reflecting increased left and right heart filling pressures and diastolic abnormalities.55 In line with this, Yilmaz et al. observed that CA125 concentrations were negatively correlated with LVEF and positively correlated with systolic pulmonary artery pressure in a retrospective study that included 150 patients with chronic and acute HF.56 In addition, the authors of that study showed that the presence of systolic dysfunction, right ventricular dilation and pericardial effusion were independent predictors of elevated CA125 concentrations.56
Finally, Vizzardi et al. showed that CA125 was positively correlated with parameters of diastolic and systolic function of the left ventricle, such as the E wave of Doppler mitral flow, the E/A ratio, isovolumic relaxation time, deceleration time and the myocardial performance index, in a cohort of 200 chronic HF patients with idiopathic or ischemic cardiomyopathy.57 Very recently, Núñez-Marín et al. demonstrated that CA125, but not NT-proBNP, was significantly associated with congestive intrarenal venous flow patterns measured by Doppler ultrasound in patients with acute HF, thus indicating renal congestion.58 Unfortunately, to date, there are still no studies relating the findings of lung ultrasonography and bioimpedance to serum CA125 concentrations, and this is the avenue that should be explored in the future.
CA125 as a Prognostic Factor of Mortality and Readmissions Due to Heart Failure
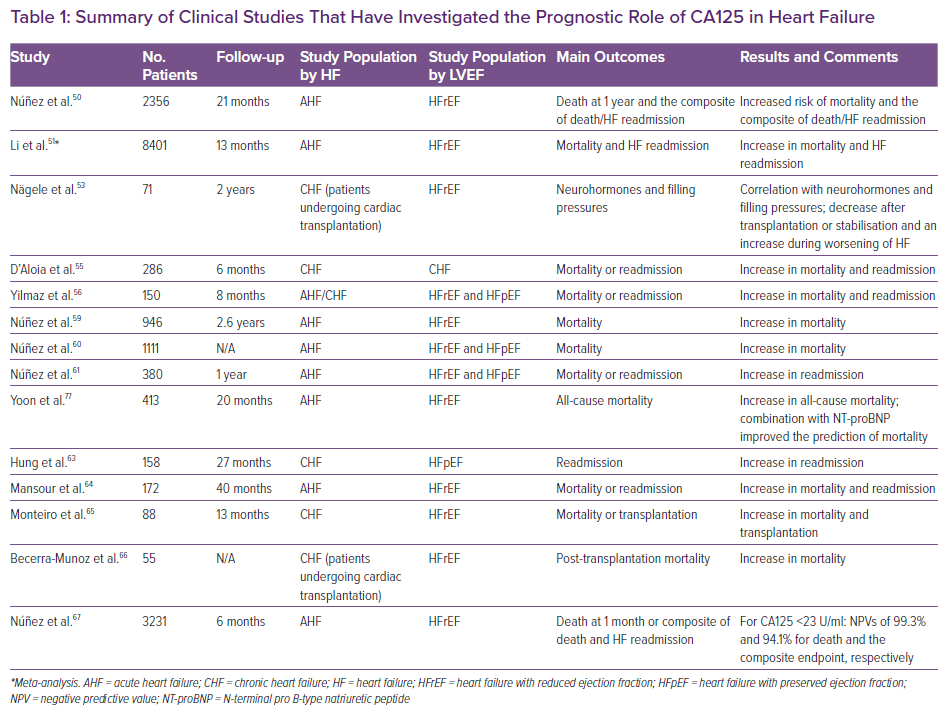
Many study groups investigated the prognostic role of CA125 in patients with acute and chronic HF in terms of death and HF readmission in various clinical settings.44,47,51,53,55,56,59–67 Apart from a randomised clinical trial by Núñez et al. (CHANCE-HF), most of the studies were designed as observational studies, and they largely included patients with acute HF.61 In addition, only one study reported on the prognostic role of CA125 in patients with HF with preserved ejection fraction (HFpEF) exclusively.63 The authors of that study highlighted that, in women with HFpEF, CA125 may aid in the prediction of HF hospitalisations, especially if used in addition to standard NT-proBNP measurement.63 It is important to highlight that all the studies in question showed a positive correlation between CA125 concentrations and adverse events such as death or hospitalisation due to HF.
Furthermore, it has been shown in many studies that CA125 remains a significant prognostic predictor of poor prognosis, even after adjustment for the relevant baseline covariates (sex, age, previous hospitalisations, systolic blood pressure, heart rate, renal function, pleural effusion, atrial fibrillation, LVEF, sodium plasma concentrations, in-hospital treatment and natriuretic peptide levels), thus bringing further evidence to the real-life clinical utility of this ‘cancer biomarker’ in the setting of HF.
Finally, a recent subanalysis of the BIOSTAT-CHF (Biology Study to Tailored Treatment in Chronic Heart Failure) study and the validation cohorts indicated that CA125 is strongly associated with a higher risk of 1-year all-cause mortality and the combined endpoint of all-cause death and hospitalisation for HF.47 Apart from including the largest number of patients, this study stands out because it confirmed the prognostic value of CA125 independent of traditional confounders and, more importantly, highlighted the value of CA125 as a biomarker for risk stratification in HF independent of traditional symptoms and signs of congestion. Of note, the association between CA125 and adverse events remained significant in patients with mild, moderate and severe congestion, paving the way for the incorporation of CA125 into multiparametric congestion scores. Importantly, a clinical pearl that needs to be addressed is the fact that increased CA125 in the setting of HF may preclude its use as a biomarker of ovarian cancer. Accordingly, the newly established role of CA125 in HF should also be underscored in the gynaecological literature.
The most recent study investigated what is the best laboratory cut-off point for the identification of patients who are least likely to experience 1-month death or the composite of death and HF readmission after being discharged for the index acute HF event.67 That study showed that among patients admitted with acute HF, CA125 concentrations <23 U/ml identified a subgroup of patients at low risk for adverse events who may not require intense post-discharge monitoring, with a negative predictive value of 98.6% for death and 96.6% for the composite endpoint during the 30 days after discharge.67 It is important to note that these results were derived from a large cohort of 3231 patients with acute HF, and were then externally validated in a cohort of patients hospitalised in the BIOSTAT-CHF study (n=1583). The studies that have investigated the prognostic role of CA125 in HF are summarised in Table 1.
CA125 as a Therapeutic Guide in Heart Failure
Decongestion therapy represents the cornerstone of HF management.68 Despite diuretics being the mainstay of treatment for volume overload in HF patients, no randomised trials have shown the mortality benefits of diuretics in HF patients, and the most effective diuretic titration strategies in this population are a question of debate.69 One of the most interesting properties of CA125 is its potential for monitoring and guiding decongestion treatment in the setting of acute HF.43,44,47,59 Specifically, according to several studies, plasma CA125 concentrations change in parallel to changes in the clinical status of HF patients.52,53,55 Moreover, Núñez et al. consecutively measured CA125 concentration trajectories in patients with acute HF and demonstrated that within the first month after hospital discharge, CA125 concentrations decreased towards normal in the subset of patients with lower risk.59 However, in patients in whom the CA125 concentrations remained high, there was an increased risk of all-cause mortality after the decompensation episode.59 Based on these results, it could be argued that CA125 is a biomarker that reflects the degree of congestion resolution in patients with acute HF, thus implicating its potential role in tailoring decongestion treatment.
Recently, the interest in the use of biomarkers to guide the course of decongestive treatment increased significantly. The main premise is to optimally escalate treatment in patients with volume overload (especially with residual congestion and/or diuretic resistance), but, more importantly, to reduce diuretic dosage in patients who would not benefit from this treatment modality, thus alleviating potential adverse effects. Nevertheless, studies that tried to use NT-proBNP for this purpose yielded heterogeneous and rather disappointing results, because the efficacy of guiding treatment by measuring NT-proBNP concentrations showed neutral results in terms of hospitalisations and cardiovascular mortality compared with the conventional strategy of decongestion treatment.70–73
Conversely, CA125 has shown promising properties for guiding treatment following an episode of acute HF. In a pivotal multicentre trial that included 380 patients with acute HF (CHANCE-HF), the authors sought to compare CA125-guided therapy, characterised by titrating the diuretic and statin dose, as well as modifying monitoring frequency, with standard of care (SOC) in terms of the composite outcome of 1-year death and acute HF readmissions.61 In that study, compared with SOC, CA125-guided therapy resulted in a significant reduction in the time to the first event and recurrent events at 1 year of follow-up.61 However, although the effect was attributed to better individualisation of patients’ decongestion treatment, it was mostly driven by the significant reduction in rehospitalisations (51%) without an effect on mortality. In addition, in patients assigned to the CA125-guided strategy, titration and clinical monitoring visits were much more frequent, and prescriptions of statins were notably increased (30%), raising doubts concerning the cost-effectiveness of such a strategy.61
Furthermore, in a recent review, the same authors proposed a modus operandi for using CA125 in tailoring decongestion treatment, which they put into practice in the aforementioned CHANCE-HF study.49 According to their suggestions, CA125 concentrations should be measured in each episode of HF decompensation and during outpatient visits following HF hospitalisation. Given that the half-life of CA125 ranges from approximately 5–7 to several days, the authors argue that, in most cases, it is sufficient to determine an initial value during the index hospitalisation, whereas in patients hospitalised for a longer time, serial measurements of CA125 could provide incremental clinical value.74,75 Conversely, there is currently no evidence to support the routine determination of CA125 in successive outpatient visits of stable patients without evidence of recent HF decompensation.
Núñez et al. proposed a value of 35 U/ml CA123, defined by the commercial reagent, as a diagnostic threshold because it has been shown that this cut-off value provides robust discrimination between patients with better and worse prognoses.61 For patients in whom CA125 concentrations fall below the threshold of 35 U/ml at the first outpatient visit after hospitalisation for HF decompensation, less intensive diuretic management is advised, especially among patients who may receive an equivalent dose of furosemide ≥120 mg/day. Patients at intermediate risk, namely those who exhibit a 25% reduction in CA125 concentrations but without its ‘normalisation’ (i.e. CA125 >35 U/ml), should be followed more closely because there is a high probability that these patients will require intensification of diuretic treatment.
Similarly, the addition of an aldosterone antagonist is advised or an increase in the total daily diuretic dose if furosemide equivalent <80 mg/day is prescribed to these patients. Finally, among patients with persistently high or rising CA125 concentrations following a decompensation event, clinical escalation of diuretic treatment is advised, with an increase in the dose of loop diuretics and/or the addition of hydrochlorothiazide, chlortalidone or aldosterone antagonists, or the administration of intravenous furosemide. Similarly, a shorter outpatient follow-up period for these patients is advised and should be scheduled for 1–4 weeks.
Of important note, using the proposed algorithm, Núñez et al. recently conducted an open-label randomised study in which the utility of the CA125-guided diuretic strategy was evaluated in patients with acute HF and renal dysfunction.76 That trial provided promising data, because the CA125-guided strategy significantly improved the estimated glomerular filtration rate and other parameters of renal function at 72 h. Furthermore, patients in the active arm with CA125 concentrations >35 U/ml received the highest furosemide equivalent dose and had higher diuresis compared with the usual-care group.76
Implementation of the Use of CA125 in Clinical Practice
Based on current findings, we can suggest that CA125 should be measured at the time of admission of patients with HF decompensation. Of note, because CA125 is not a cardiac-specific biomarker, its circulating concentrations should not be exclusively interpreted as a proxy of congestion. Measurement of CA125 concentrations should be complemented with clinical information obtained from physical examination, natriuretic peptide measurement and echocardiographic findings (e.g. the diameter of inferior vena cava, LUS, ultrasound examination of third-space fluids and extravasations, among others). Such findings, especially if concordant with CA125 concentrations, would help determine the cause and degree of congestion. Therefore, initially high CA125 concentrations may inform clinicians that the patient is at an increased risk of short-term adverse events compared with their counterparts who have normal CA125 concentrations. As highlighted previously, this may be particularly applicable for older patients with impaired renal function and predominant involvement of the right side of the heart, as well as for patients with subclinical congestion.
Moreover, high CA125 concentrations may guide the clinician to use intensive decongestion strategies with more stringent monitoring, because this subgroup of patients may benefit from a more aggressive diuretic approach. Apart from prognostic assessment and choice of volume management strategy, some clinical value may be gained from serial measurements of CA125, because normalisation of CA125 and its lowering below the 35 U/ml threshold is the pattern that occurs most frequently and is associated with clinical improvement and a lower risk of adverse clinical events, regardless of the initially measured value. In this regard, it is critical to address the dynamics of the biological half-life of CA125. Namely, owing to the long half-life of CA125, serial measurements during the first days following a hospitalisation would be practically useless for capturing information about acute response to therapy. Because HF-related hospitalisations often last longer than 7 days, it would probably be a reasonable approach to measure CA125 concentrations at the time of admission for HF and at least 7 days after the initial measurement or near hospital discharge. With that said, a pattern of weekly kinetics of CA125 following decompensation could be a promising tool for monitoring decongestion efficacy among patients with HF, and would also be useful for risk stratification.
Moreover, demonstrated by Yoon et al., patients with high CA125 but low NT-proBNP concentrations during hospital admission for acute decompensated HF have worse mid-term prognosis than patients with low CA125 and low NT-proBNP concentrations.77 Similarly, those with high NT-proBNP and high CA125 concentrations had the worst prognosis, and CA125 was found to be an independent factor associated with all-cause mortality in this population.77 Thus, the body of accumulating evidence suggests that the combined use of CA125 and NT-proBNP may be superior for risk estimation in this population than the conventional use of NT-proBNP alone. In the years to come, further exploration will hopefully unravel the pathobiological role of CA125 in HF and answer whether CA125-guided decongestion strategies would have an effect on hard clinical endpoints, such as mortality.
Conclusion
Emerging data suggest that CA125 has the potential to be integrated into the daily clinical work-up of patients with HF, not only as a prognostic indicator, but also as a marker reflecting congestion (severity) status and as a guide in tailoring decongestion treatment. Several important points justify the role of CA125 for this application. First, at this point, there are no biomarkers in routine clinical practice that reflect the congestive status of patients with HF. Second, there is a substantial pathophysiological background supporting the role of CA125 in this setting. Third, emerging data show that CA125 provides additional prognostic information beyond classical biomarkers in HF, specifically NT-proBNP, and CA125 has proven to be a valuable guide in tailoring diuretic treatment. Fourth, unlike NT-proBNP, CA125 does not appear to be significantly modified by anthropometric factors, such as age, weight or renal dysfunction, and given, its long half-life, plasma CA125 concentrations remain stable, improving its prognostic utility. Finally, the well-established and widely adopted use of CA125 as a biomarker in ovarian cancer for several decades has led to the wide availability and low cost of CA125 measurement, because this biomarker can be readily measured in most clinical centres and hospitals worldwide.
Gaps in our full understanding of the molecular background, the speculative nature regarding the role of CA125 in HF progression, the lack of an optimal cut-off value and the lack of definitive data from large multicentre studies represent the main limitations that currently preclude the use of CA125 in HF. Hence, future well-designed, large-scale studies are needed to fully establish and validate the purpose, exact timing of measurement and effect of CA125-guided management in HF on relevant clinical endpoints, such as cardiovascular and all-cause death and HF-related hospitalisations.










