With this letter, the authors would like to express their views to the Editor-in-Chief on the use of extracorporeal membrane oxygenation (ECMO) as a treatment for branch pulmonary artery rupture following right heart catheterisation (RHC). Iatrogenic pulmonary artery injury is a rare, but potentially life-threatening, complication of RHC, associated with high mortality due to limited options for acute management. The authors would like to highlight acute considerations in response to iatrogenic pulmonary artery injury after RHC, and identify an effective therapy in veno-arterial ECMO (VA-ECMO) that may help to stabilise patients acutely by decompressing the right ventricle and pulmonary circulation.
We present the case of a 64-year-old woman with dilated non-ischaemic cardiomyopathy, chronic kidney disease and a history of ventricular tachycardia, who was referred for evaluation of candidacy for advanced heart failure (HF) therapies. She was diagnosed with HF in 2006 and had remained clinically stable on guideline-directed medical therapies until 2017, at which time HF symptoms became rapidly progressive and she had multiple HF-related admissions.
Informed consent was obtained from the patient for the presentation of the patient’s data in the current manuscript, and no identifiable information is included. We declare that this work was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
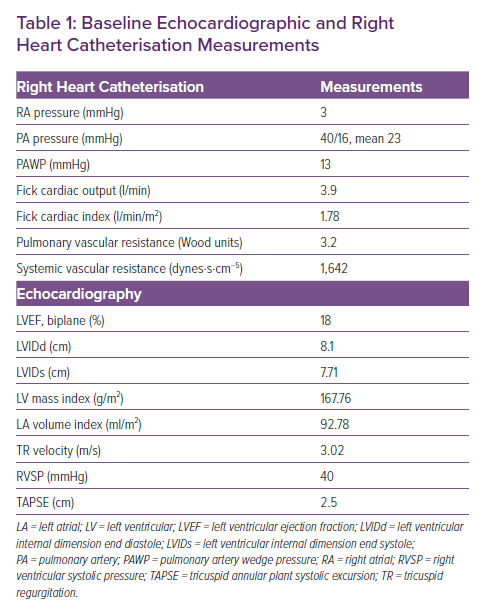
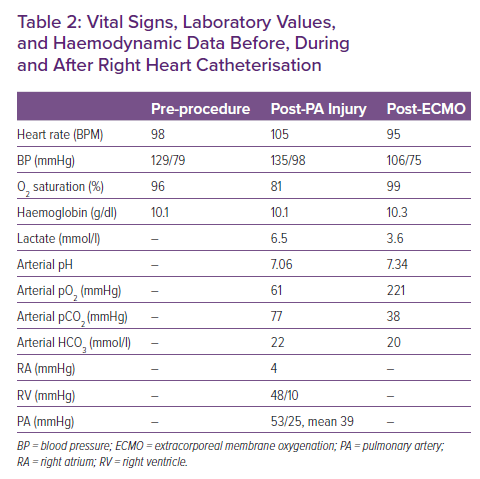
At the time of her evaluation, echocardiogram was notable for a severely dilated left atrium and left ventricle, with severely depressed function, normal right ventricular function and severe secondary mitral regurgitation (Table 1). She underwent RHC, which demonstrated mild pulmonary hypertension, low cardiac output with a cardiac index of 1.78 l/min/m2, normal pulmonary arterial wedge pressure, elevated pulmonary vascular resistance and elevated systemic vascular resistance (Table 1). Despite attempts to optimise medically with afterload reduction, the patient remained symptomatic and returned for RHC 2 weeks later with a plan for nitroprusside challenge to evaluate the reversibility of her pulmonary hypertension. Pre-procedural vital signs were normal (Table 2) and cardiac telemetry showed an atrial-sensed, biventricular-paced rhythm. A pulmonary artery (PA) catheter was successfully inserted via the right internal jugular vein, and advanced with the balloon inflated under fluoroscopic guidance through the right atrium, right ventricle and into the PA. Her right atrium pressure was 4 mmHg, and PA pressure was 53/25 mmHg with a mean of 39 mmHg. With deflation of the balloon, the catheter spontaneously advanced into the right posterior basal pulmonary artery and was rapidly withdrawn to the proximal PA without resistance. Almost immediately, the patient began coughing and had new-onset haemoptysis consistent with presumed iatrogenic branch PA injury.
Under fluoroscopic guidance, the PA catheter balloon was re-inflated and advanced to the site of the suspected injury in an attempt to tamponade the location of the bleeding. The patient was then placed in the right lateral decubitus position, allowing the injured right lung to be in the dependent position. Femoral arterial and venous access were obtained, and fluid resuscitation was begun and blood ordered. Given the inability to control bleeding, leading to airway compromise, left main stem bronchus intubation was performed, followed by double-lumen endotracheal intubation. Despite stable haemoglobin, heart rate and blood pressure, the patient developed a combined respiratory and metabolic acidosis within 20 minutes of branch PA injury (Table 2), and the decision was made to perform peripheral cannulation for VA-ECMO. Notably, heparin was not administered with large bore ECMO cannulation due to ongoing bleeding. With the initiation of ECMO, the patient’s haemoptysis decreased significantly, with subsequent improvement in oxygenation and acidosis (Table 2).
Bronchoscopy was performed through the right endotracheal tube lumen, and approximately 150 ml of blood and clots was suctioned out of the right lung. The patient stabilised on VA-ECMO support over the next 18 hours, and repeat bronchoscopy showed resolution of active bleeding 12 hours after the PA injury. The patient was able to be weaned off inotropic support and decannulated from ECMO. Despite her complicated course, she required only 2 units of packed red blood cell transfusion with a haemoglobin nadir of 9.2 mg/dl, renal function was stable with a creatinine level of 1.3–1.5 mg/dl and estimated glomerular filtration rate of 40–50 ml/min/1.73 m2, and she remained neurologically intact. She was treated with antibiotics for possible hospital-acquired pneumonia versus pneumonitis caused by the PA injury. The patient was ultimately restarted on guideline-directed medical therapies for HF, and discharged to complete rehabilitation therapy. She returned to complete her advanced HF therapy evaluation and was approved for transplant listing, with plans for a left ventricular assist device as a bridge to transplant.
Discussion
Measurement of invasive haemodynamics via RHC is a common procedure for both the diagnosis and management of patients with advanced HF, and is a guideline-recommended procedure for evaluation for heart transplant.1 While generally considered to be a low-risk procedure, PA catheters may lead to complications, including arrhythmia, pneumothorax, heart block, lung infarction, thrombosis, air embolism (either through entrainment of air in the catheter or balloon rupture), infection, catheter knotting and pulmonary artery damage.2
Although branch PA injury is a complication of RHC that is estimated to only occur in approximately 0.5% of cases, mortality associated with this event is as high as 70%.2,3 Risk factors for PA injury during RHC include the presence of pulmonary hypertension, concomitant anticoagulant therapy, advanced age, mitral valve disease and hypothermia.4
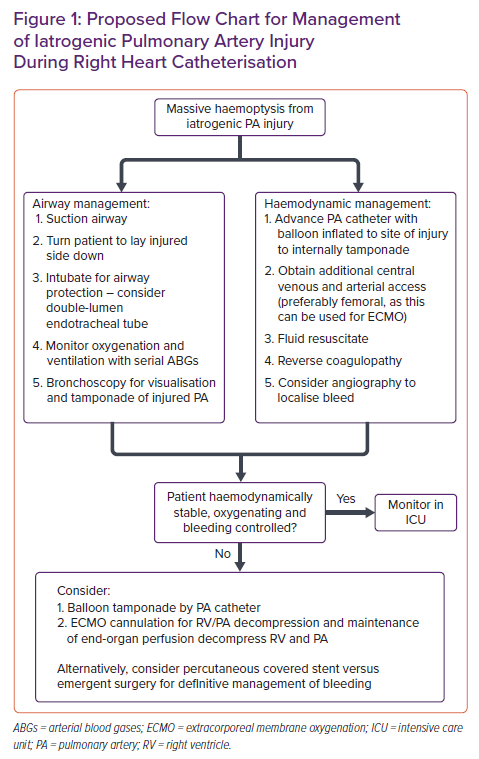
Management of branch PA injury requires immediate action, as acute massive haemoptysis will compromise the airway and may quickly lead to haemodynamic instability (Figure 1). Initial management includes identifying the side of bleeding and positioning the patient with the injured lung down. The next step is to establish an airway via either unilateral lung ventilation (through selective contralateral main stem intubation) or double-lumen endotracheal intubation (to allow separate control of both lungs). Accurate measurement of oxygenation is a crucial determinant of stability, and can be accomplished with serial arterial blood gases. The next step is to ensure adequate venous and arterial access to allow for fluid and blood resuscitation, as well as invasive blood pressure monitoring. If allowable either through the existing PA catheter or through a separate catheter, angiography can be considered to localise the site of bleeding. Finally, reversal of any coagulopathy is necessary, and attempts should be made to control the site of bleeding. Options include endobronchial management with mechanical tamponade or administration of procoagulants, percutaneous balloon tamponade or covered stent placement, emergent surgery and VA-ECMO.5–7 While cannulation of VA-ECMO occurred from femoral sites in our case, the existing internal jugular sheath can also be exchanged for a short, large venous cannula with inflow into the ECMO circuit.
ECMO has been shown to be a life-saving strategy to promote recovery of lung injury, but its utility in the acute treatment of massive haemoptysis has largely only been reported in postoperative management of haemorrhage following pulmonary endarterectomy and patients already being supported by VA-ECMO.8,9 In both the cases of post-endarterectomy or pulmonary haemorrhage while on VA-ECMO support, maximising support through the reduction in blood flow via the right ventricle and pulmonary circulation allowed for successful recovery.8,9 While heparin administration is generally required for large-bore ECMO cannulation, heparin administration in our case was contraindicated due to ongoing bleeding. At higher flow rates, ECMO circuits can be placed without heparinisation, but carry a risk of spontaneous clotting of the circuit. Despite this risk, our case report highlights that early deployment of VA-ECMO may additionally be a valuable treatment in the management of PA catheter-associated iatrogenic PA injury.
VA-ECMO decreases blood flow through the right ventricle and pulmonary circulation by directly removing blood from the right atrium and re-directing it into the aorta after oxygenation. This strategy accomplishes two critical goals in cases of PA rupture. First, it allows for control of bleeding through a reduction in pulmonary blood flow. Second, it preserves adequate circulation to the rest of the body. While these potential benefits must be weighed against the known risks of ECMO, including bleeding and coagulopathy, ECMO may be an effective strategy to stabilise patients with PA injury and massive haemoptysis after having failed more conservative measures (Figure 1).10
In summary, we report the case of a patient with advanced non-ischaemic cardiomyopathy who suffered PA injury due to PA catheterisation and survived after being placed on VA-ECMO. Given the extremely high mortality associated with this rare, but highly lethal, complication of a commonly performed procedure, awareness of immediate management strategies, including the use of ECMO, may lead to improved outcomes.