This article explores the implications of the new European Society of Cardiology (ESC) guidelines for the diagnosis and management of heart failure (HF) from a primary care perspective.1 We specifically discuss the clinical conundrums around accurately identifying and managing patients with HF and preserved ejection fraction (HFpEF) in this context. We also consider how the latest trial evidence around treating such patients, many of whom are women, will shape contemporary and future clinical practice.
Heart Failure and Primary Care
The overall management of HF, a complex syndrome associated with poor outcomes in all its forms, remains problematic. This particularly applies in the primary care context. As with any other debilitating chronic condition, an individual’s journey with HF, while often punctuated by acute hospital episodes and premature mortality (frequently in winter), typically occurs in a community setting.2
With a broadly informed evidence base, primary care (including general practice) is designed to improve health and wellbeing, prevent disease progression, prolong life and minimise costly hospital episodes in the most vulnerable people. This must include individuals living with HF and the common clusters of other diseases (in the form of multimorbidity) that both drive and exacerbate the syndrome.3
It is from the perspective of the central importance of primary care in the optimal management of chronic HF that a review of the recently published ESC guidelines is concerning.1 These guidelines amount to more than 100 pages of expert opinion supported by 1,001 key references. Surprisingly, however, primary care is specifically mentioned in these guidelines only in respect to:
- The diagnostic value of natriuretic peptides (in the presence of the typical signs and symptoms of HF) when a threshold of 35 pg/ml for brain-natriuretic peptide (BNP) and 125 pg/ml for NT-proBNP is reached.4,5
- The general follow-up of patients with chronic HF (with no specific evidence from primary care referenced).6
While this does not necessarily mean that these and equivalent guidelines from other learned societies completely discount the role of primary care, it most probably does reflect the specialisation of HF management and a predominant focus on individuals who require acute hospital care. Notably, with few exceptions, there are no GP authors of these guidelines. This is of clinical importance, given that it has been shown in a large, real-world patient cohort that the demographic and clinical profile of people diagnosed with HF in primary care is different from that of those diagnosed in hospital, while both have equally poor 5-year survival rates.7
When one directly compares the demographic and clinical profile of the broader HF population with those recruited into contemporary clinical trials, regardless of the type of HF being treated, HF trial participants are predominantly younger, and more likely to be male and have lower levels of multimorbidity.7–10 The evidence trail and clinical experiences of those writing the guidelines are, therefore, potentially skewed away from a broader and potentially more complex primary care perspective.
Paucity of Primary Care Trials
It is worth highlighting some isolated examples of primary-care-focused HF research relevant to the two topics (diagnostic screening and long-term follow-up) and why, perhaps, few equivalent studies have been reported since.
In the STOP-HF trial, Ledwidge et al. tested the efficacy of a collaborative care model guided by BNP screening in a cohort of 1,374 individuals with cardiovascular risk recruited from 39 primary care clinics in Ireland.11 Overall, the primary endpoint of left ventricular (LV) dysfunction with or without HF occurred in 59 out of 677 (8.7%) patients in the control group versus 37 out of 697 (5.3%) patients in the intervention group (reduced risk of 45%, 95% CI [18–63%]; p=0.003).11 However, as highlighted by a recent position statement from the ESC Heart Failure Association, the primary prevention of HF has remained problematic, with no definitive role articulated for primary care in the decade since this important study was published.12
Similarly, at the genesis of multidisciplinary HF management programmes (now considered a gold-standard component in the care of HF patients discharged from hospital), Doughty et al. reported on the Auckland Heart Failure Management Study conducted in New Zealand.1,13 In a cluster randomised trial, they tested the efficacy of an integrated primary and secondary programme (including alternate GP and HF clinic visits). Unfortunately, the primary composite endpoint of death or hospital readmission within 12 months was not met.13 This contrasted with the positive results of contemporary trials of nurse-led programmes of care (particularly those with multidisciplinary teams and a component of home visits).1
Once again, therefore, there remains a vacuum in primary-care-focused HF management programmes. For example, a recent systematic review of disease management programmes in primary care revealed that most published trials did not test hard endpoints (hospitalisation or death) and focused on single disease states, such as diabetes and asthma, rather than complex conditions like HF.14 It is well established that a relatively small number of actively managed patients with complex health issues such as HF consume a disproportionate amount of healthcare resources. For example, in Australia 31–37% of patients visit their GP 4–11 times a year, and 10–14% of patients visit ≥12 times/annum.15 As Koudstaal et al. recently suggested, HF patients predominantly managed within the primary care setting have a very poor prognosis.7
Key research questions remain unanswered, such as how these high-cost/high-risk individuals can be readily identified and appropriately managed to improve their quality of life, avoid recurrent hospitalisation and premature mortality via the practical application of gold-standard therapies adapted to the skills and resources of GPs and primary healthcare teams (including pragmatic treatment uptitration and discontinuation protocols).
As the large, multicentre VIPER-BP study demonstrated, it is possible to apply decision-support tools in primary care to safely uptitrate antihypertensive therapy to improve blood pressure control in high-risk individuals and thereby reduce their future risk of HF.16 This approach applies to most if not all the main antecedents of advanced heart disease. However, the uptake of such pragmatic and proven strategies often fails when there is no funding mechanism to support their uptake.
As an alternative, the much-supported option of guideline-directed medical therapy for HF should, in theory, be easier to apply in primary care given the availability of subsidised and approved therapies.17 However, as typically occurs in the primary care HF population, this strategy is not so easily applied when a GP is faced with an ‘atypical’ patient who would have been excluded from a clinical trial because of their advanced age, type of HF (e.g. predominantly right sided) and multimorbidity.17
Overall, therefore, there is a paucity of evidence to inform the most cost-effective methods to rapidly diagnose and optimally manage HF patients in primary care.
Redefining the Syndrome
The definition of HF continues to evolve as our physiological and clinical understanding of the syndrome increases. In the latest ESC guidelines, three main phenotypes of HF are identified (all or which require the typical signs and symptoms of HF including dyspnoea and signs of congestion):
- Heart failure with reduced ejection fraction (HFrEF): characterised by a left ventricular ejection fraction (LVEF) ≤40%.
- Heart failure with mildly reduced ejection fraction (HFmrEF): characterised by a LVEF of 41–49%.
- HFpEF: characterised by a LVEF ≥50% in addition to structural cardiac abnormalities such as LV hypertrophy and/or diastolic dysfunction/impaired LV filling.1
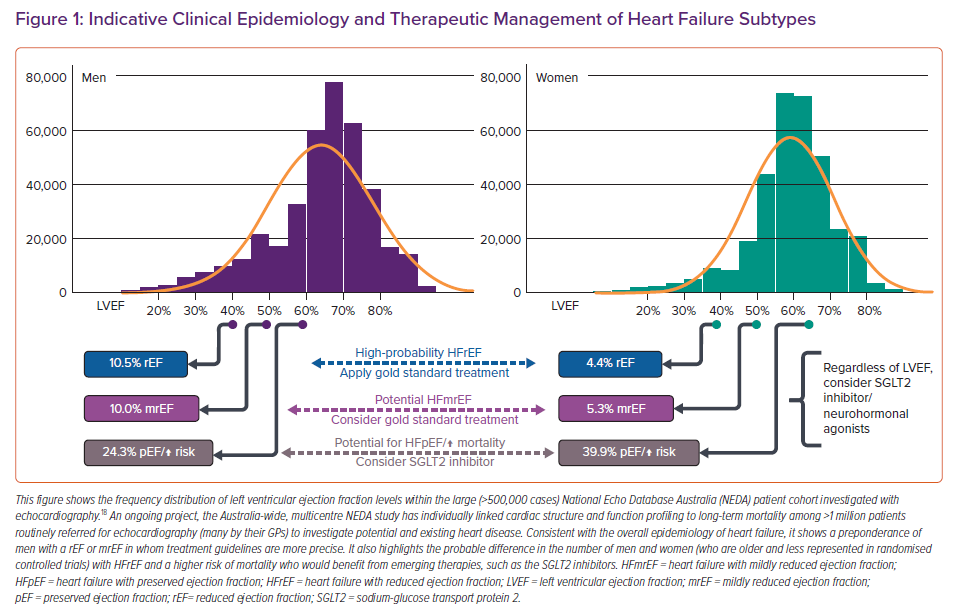
It is the last of these – HFpEF – that challenges even the most experienced physicians; it typically requires careful interpretation of an echocardiogram and the patient’s clinical profile. The NEDA study demonstrated that individuals presenting with a LVEF <65% are at increased risk of mortality (with clear sex-based differences evident given women had higher mortality rates at higher LVEF levels).18 However, identifying patients with HFpEF (LVEF ≥50%) who would benefit from the results of trials, such as PARAGON (see below) and EMPEROR-PRESERVED, based on their inclusion criteria, is challenging.8,9
For GPs contending with multiple disease states, there is a clear need to simplify the messaging around who has potentially treatable HF through a streamlined interpretation of BNP levels and subsequent echocardiographic reports, with close consideration of the funding mechanisms and cost implications within different healthcare systems. For example, in Australia, the recommended BNP screening as the firstline investigation for clinically suspected HF is not reimbursed. Furthermore, GPs need to carefully balance financial constraints with patient care. In particular, people in lower socioeconomic groups and older patients who are more susceptible to HF often cannot afford expensive investigations and multiple new medications, even if these are clinically superior.
Addressing these barriers to applying gold-standard management is becoming increasingly urgent as our understanding and evidence-based approaches to this common condition continues to evolve so rapidly.
Treating HFpEF
In recent years, the management of patients with HFrEF has been transformed with the positive results of the PARADIGM trial of angiotensin receptor-neprilysin inhibitors (ARNIs) and the EMPEROR-Reduced/DAPA-HF trials of sodium–glucose co-transporter 2 (SGLT2) inhibitors.19,20 These agents have now joined angiotensin-converting enzyme inhibitors, beta-blockers and mineralocorticoid receptor antagonists as the main treatment options for HFrEF – with a continued role for loop diuretics in patients with fluid retention and adjunctive device-based therapies in specific individuals.1
The same principles of management of HFrEF (with the caveat ‘considered’ applied in the guidelines) now largely apply to patients with HFmrEF depending on local therapeutic approvals and authorisation.1 As recently highlighted, any decline in the LVEF among this borderline group is of prognostic importance, with proactive prevention and management of coronary artery disease to preserve an individual’s LVEF critical.1,21
Consistent with a more complex definition and diagnosis algorithm, the therapeutic management of HFpEF remains difficult.1 Compared to those patients presenting with HFrEF/HFmrEF, those with HFpEF are typically women, older and have a higher burden of multimorbidity.1 This includes diabetes, hypertension, AF, chronic kidney disease and non-cardiac conditions, such as chronic lung disease.1,3 To date, management of such cases has been founded on the evidence-based treatment of these conditions rather than of HFpEF per se.
Despite strong evidence of the efficacy of ARNIs and other neurohormonal-modulating therapies in improving outcomes among those with LVEF indicative of HFmrEF (LVEF 40–49%) – as demonstrated by careful analyses of the PARAGON-HF trial and the combined trial evidence – HFpEF has proven to be a graveyard for HF therapies and patients alike.8,22 However, the emergence of SGLT2 inhibitors has finally offered evidence-based, therapeutic options for these patients.20 First, independent of an individual’s LVEF, SGLT2 inhibitors have been proven to reduce clinical events in patients with diabetes, those at similarly high-risk of experiencing a cardiac event, chronic kidney disease, those with an established form of cardiovascular disease and with a history of hospitalisation for HF (all characteristics of HFpEF).20
Careful analyses of the impact of neurohormonal antagonists according to an individual’s LVEF and the recently completed EMPEROR-Preserved trial provide both encouragement and caveats to the effective scope and treatment of HFpEF.9,22 In this ground-breaking RCT, the efficacy of SGLT2 inhibitor empaglifozin was tested in 5,988 patients with New York Heart Association class II–IV dyspnoea and an LVEF >40%.15 Overall, the SGLT2 inhibitor was associated with a significant 21% reduction (actual rates 13.8% versus 17.1%) in the composite primary endpoint of cardiovascular death or hospitalisation for HF during median 26-month follow-up. The impending results of the equivalent DELIVER trial, which is examining the potential benefits of dapagliflozin therapy among a similar patient cohort, will further clarify the role of SGLT2 inhibitors in treating HFpEF.23
This does not tell the whole story, however. Serious adverse events were reported in around 50% of EMPEROR-Preserved participants with similar rates reported in both treatment arms and treatment being discontinued in 18.4–19.1% of participants. In the treatment arm, there was an increased rate of genital and urinary tract infections and episodes of hypotension.9 Such events would invariably be reported to and managed by a GP in routine clinical practice. Thus, as with any new agent or clinical indication in HF, there is an inherent expectation on primary care to consider and manage complicated issues around the benefits versus risks of continuing agents, such as the SGLT2 inhibitors, while attempting to interpret trial evidence derived from typically younger, less complicated patients.17
As recently posed by Petrie et al. before the EMPEROR-Preserved trial results were reported, the key question is: do SGLT2 inhibitors work across the entire spectrum of HFpEF?20 Critically, the probable answer is a qualified ‘no’. In a prespecified sub-analysis of the EMPEROR-Preserved trial, the greatest benefits occurred in those with an LVEF <50% (HR 0.71, 95% CI [0.57–0.88]) and 50–59% (HR 0.80, 95% CI [0.64–0.99]), but not entirely for those with an LVEF >60% (HR 0.87; 95% CI [0.69–1.10]).9 Moreover, consistent with sex-specific thresholds of mortality at more preserved levels of LVEF, women appeared to derive the greatest benefits from the SGLT2 inhibitor (25% versus 19% hazard reduction in the primary endpoint).22
Assimilating all the available data (from the clinical epidemiology of LVEF levels and associated mortality to contemporary HF guidelines and emerging trial evidence) is not easy from a primary care perspective. Figure 1, based on the distribution of LVEF observed in the large National Echo Database Australia cohort, provides a broad summary of how the spectrum of HF cases (including those with HFpEF) might be managed from a primary care perspective when considering: the latest ESC classification of HF and the current evidence in favour of neurohormonal blockade according to LVEF levels.1,22
New Solutions for an Old Problem
Despite the encouraging results of the EMPEROR-Preserved trial and attempts to simplify the classification of HF overall, the clinical conundrums posed by HFpEF remain.1,15 This is particularly true for GPs and primary care teams, who continue to interact with and manage those with a potential or established diagnosis of HFpEF. Current guidelines such as those produced by the ESC provide little direction in this regard.1 There is a clear need to rectify this through a specific interpretation of recommendations and pragmatic clinical algorithms that are relevant to primary care. Any such recommendations would need to be supported by dedicated academic detailing and evidence-based translation programmes in the primary care setting.
This is even more urgent with the emergence of novel therapeutic agents, such as the SGLT2 inhibitors. In this context, there is also potential to develop better lines of communication and levels of trust between GPs and cardiologists. This could include easier access to echo investigations and informative diagnoses for GPs to ensure that evidence-based therapeutics are appropriately prescribed, modified and managed in high-risk patients with all forms of HF.
Moreover, renewed funding of research focusing on primary care is urgently required to address the critical lack of evidence to guide the optimal management of the growing number of older people with HFpEF and, typically, high levels of multimorbidity who experience poor health outcomes.3