Nearly one-half of heart failure patients have heart failure with preserved ejection fraction (HFpEF), and the prevalence appears to be rising.1 Today, HFpEF represents the most common cause of hospitalisation for heart failure, surpassing heart failure with reduced ejection fraction (HFrEF).2 Patients with HFpEF experience similar patterns of morbidity and functional decline as those with HFrEF, but few effective treatments are available.3 The effects of medical management in patients of HFpEF are very limited, mainly focusing on treatment of heart failure symptoms and comorbidities. Large clinical trials investigating the effects of candesartan, irbesartan, perindopril, spironolactone and, more recently, sacubitril–valsartan in patients with HFpEF all failed to reach their primary endpoints.4–8 Thus, there is a growing need for the introduction of novel treatment strategies that could potentially improve the outcomes in this large patient population.
Potential Targets for Cell Therapy in HFpEF
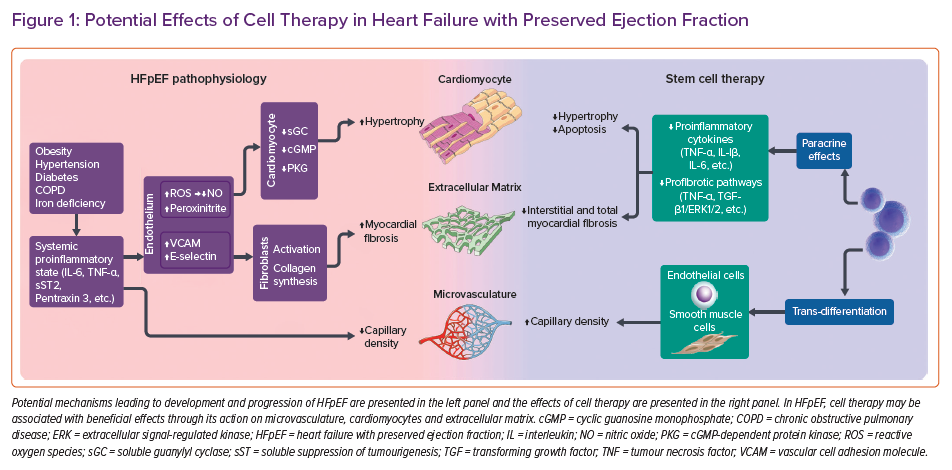
Currently, the central paradigm for the pathophysiology of HFpEF is based on the hypothesis that comorbidities lead to a systemic proinflammatory state and coronary microvascular endothelial inflammation.9 Patients with HFpEF have a high prevalence of co-morbidities such as obesity, diabetes, hypertension or renal dysfunction, which can induce the systemic proinflammatory state. In this proinflammatory state, coronary microvascular endothelial cells produce reactive oxygen species, which limits nitric oxide (NO) bioavailability. Reduced NO signalling from dysfunctional endothelium influences adjacent cardiomyocytes and cardiac fibroblasts via the soluble guanylyl cyclase–cyclic guanosine monophosphate–cGMP-dependent protein kinase pathway, resulting in functional and structural cardiac changes such as delayed myocardial relaxation, increased cardiomyocyte stiffness, cardiac hypertrophy and interstitial fibrosis.10 In addition, NO imbalance affects endothelial progenitor cells (EPCs), leading to impaired endothelial repair and regeneration.11
EPCs are bone-marrow-derived circulating cells able to proliferate and differentiate into functional mature endothelial cells. EPCs are mobilised into the circulation in response to tissue or vessel injury and incorporate into sites of injury. Circulating EPCs can be evaluated by measuring the expression of various surface antigens, including CD34, CD133 and vascular endothelial growth factor receptor 2.12 In ischaemic conditions, EPCs are responsible for the formation of new vessels via direct incorporation into the newly developing vasculature and the production and secretion of angiogenic cytokines.13
Levels of circulating EPCs are significantly reduced in patients with HFpEF and the remaining EPCs have impaired function.12 Furthermore, the numbers of circulating EPCs have been shown to inversely correlate with the degree of diastolic impairment.14 This is in illustrated by findings from autopsy studies demonstrating that HFpEF patients have lower coronary microvascular density and more severe fibrosis than control subjects regardless of the severity of epicardial coronary disease.15 In these subjects, the severity of myocardial fibrosis was inversely associated with microvascular density.
More recently, it has been shown that patients with HFpEF have a very high prevalence of microvascular dysfunction, demonstrated by reduced myocardial flow reserve at single-photon emission CT, lower coronary flow reserve, and a higher index of microvascular resistance.16 Together, this evidence strongly supports the hypothesis that coronary microvascular endothelial inflammation in HFpEF may be the key factor leading to impaired angiogenesis, microvascular rarefaction and myocardial fibrosis.
Mechanisms of Action of Cell Therapy in Heart Failure
Different populations of autologous and allogeneic stem cells have been studied in either preclinical or clinical settings of chronic heart failure for their capacity to repair and/or regenerate the failing myocardium.17 Initially, the main reparative mechanism of cell therapy was thought to be a direct replacement of damaged cardiomyocytes with new, cell-derived cardiomyocytes through a process of trans-differentiation.18 While this mechanism has been demonstrated in preclinical models of heart failure, it has never been unequivocally confirmed in the clinical setting. Based on the current evidence, it is believed the main reparative mechanisms of cell therapy on the failing myocardium are mediated through paracrine effects that affect myocardial neurohumoral activation, inflammation, fibrosis, apoptosis, Ca2+ handling and metabolism, stimulation of neovascularisation and activation of endogenous cardiac-resident cells.19,20 These mechanisms may be associated with beneficial effects in HFpEF through improved angiogenesis, decreased fibrosis and reduced inflammation (Figure 1).
Effects of Cell Therapy on Angiogenesis
Current data suggest that cells transplanted into the failing myocardium likely stimulate angiogenesis and may thus significantly improve myocardial regional perfusion. Kawamoto et al. demonstrated that intramyocardial injections of CD34+ cells are associated with a significantly increased myocardial capillary density in an animal model of heart failure.21 The authors additionally showed that the application of a single cell type (in this case CD34+) may be more advantageous over unfractionated bone marrow mononuclear cells because the latter might cause detrimental changes to the myocardium (haemorrhagic necrosis), thus offsetting the potential benefits of cell therapy.21
In accordance with these findings, Schuleri et al. demonstrated that intramyocardial injections of mesenchymal stem cells (MSC) likely improve myocardial perfusion (estimated with cardiac MRI) in a preclinical model of ischaemic heart failure.22 These encouraging preclinical findings were subsequently confirmed in a clinical trial in patients with dilated cardiomyopathy, where a significant improvement in myocardial perfusion six months after intracoronary CD34+ cell injections was found.23 Of note, these changes in myocardial perfusion correlated with a significant improvement in contractile performance of the failing myocardium, a decrease in neurohumoral activation, improved exercise capacity and improved overall survival at 5 years follow-up.24
In patients with ischaemic heart failure, intramyocardial MSC injections were associated with an improvement of regional perfusion in the injected segments of the failing myocardium, which translated to improved contractility of these segments. Of interest, surgically revascularised segments that were not treated with cell injections did not functionally improve to the same degree.25 These data suggest that the changes in myocardial perfusion after cell therapy appear to occur independently from the status and progression of coronary artery disease.25 Moreover, since the changes in perfusion after cell therapy have also been demonstrated in patients with dilated cardiomyopathy and normal coronary arteries, it is likely that factors other than coronary atherosclerosis may be responsible for the observed changes in myocardial perfusion.23 Evidence suggests that cells may exert their beneficial effects on myocardial angiogenesis through the paracrine secretion of bioactive growth factors, such as vascular endothelial growth factor and fibroblast growth factor.26,27 It has been suggested that stem-cell-derived paracrine factors may further trigger a secretion of other paracrine factors from the host myocardium thus potentiating their effect. This hypothesis may partly explain the apparent discordance between significant clinical effects on the remodelling process caused by a limited number of surviving stem cells in the host myocardium.28
Effects of Cell Therapy on Extracellular Matrix
In addition to exerting positive effects on the microvascular homeostasis, cell therapy has also been associated with reverse remodelling of the extracellular matrix (ECM) in the setting of chronic heart failure. Preclinical data suggest that cell therapy may be associated with a significant reduction in myocardial fibrosis.18,29 These findings have been further corroborated by clinical data, where the intracoronary infusion of cardiosphere-derived autologous stem cells (CDCs) was shown to be associated with a 42% reduction in myocardial scar burden (as assessed by cardiac MRI), and an increase in myocardial viability and regional contractility 12 months after the procedure.30 In accordance with these findings, intramyocardial injection of autologous MSCs was also associated with significant (48%) reduction in myocardial scar burden, improved myocardial perfusion and increased contractile performance.25
Although the exact pathophysiological mechanism of stem cell action on ECM reverse remodelling remains to be clarified, currently available data suggest that stem cell therapy may affect myocardial scar by inhibiting tumour necrosis factor (TNF)-α and the transforming growth factor-β1/extracellular signal-regulated kinase 1/2 fibrosis pathways and by the direct actions on resident fibroblasts. The latter may result in decreases in transcript levels of matrix metalloproteinase (MMP)-2, MMP-7, and MMP-9; collagen I and collagen III and tissue inhibitor of metalloproteinase-1, thereby normalising the turnover of ECM proteins.31
Effects of Cell Therapy on Myocardial Inflammation
Cell therapy has also been shown to dampen the pro-inflammatory milieu in the failing myocardium by downregulating the expression of proinflammatory cytokines, such as TNF-α, interleukin (IL)-1β, IL-6 and monocyte chemo-attractive protein.32 Furthermore, stem cells (especially MSCs) have been demonstrated to possess immunomodulatory properties that are likely exerted through cell-secreted paracrine factors and direct cell-to-cell interactions, which may affect a wide range of cells involved in the pro-inflammatory response.33 It is further suggested that cell-derived paracrine factors may activate tissue macrophages, which promotes structured angiogenesis and induces a switch from pro-inflammatory M1 phenotype to anti-inflammatory M2 phenotype, most likely via insulin-like growth factor-1 and IL-10 pathways.34,35 Collectively, these data suggest that the anti-inflammatory properties of cell therapy may have a significant role in stimulating the process of reverse remodelling of the failing myocardium.
Preclinical Evidence for Cell Therapy in HFpEF
In contrast to the abundant preclinical evidence on the effects of cell therapy in HFrEF, data on the effects of cell therapy in HFpEF models are very scarce. Using a hypertensive rat model of HFpEF, Gallet et al. investigated the effects of CDCs on left ventricular structure and function.36 At 13–14 weeks of age the rats were randomly allocated to receive intracoronary infusion of either allogeneic CDCs (n=24) or placebo (n=24). Follow-up lasted for 4 weeks after randomisation. Before randomisation and at 4 weeks after treatment, echocardiography and invasive haemodynamic measurements were performed. At the end of the follow-up, CDC therapy was associated with a decrease in E/A ratio and halted left atrial enlargement. The results of haemodynamic measurements demonstrated a twofold higher end-diastolic pressure in placebo-treated animals when compared to those receiving CDC therapy. Furthermore, CDC therapy was associated with decreased lung congestion and improved survival. The histological analysis of the myocardium demonstrated increased capillary density, decreased inflammation and decreased fibrosis in CDC-treated animals. CDC treatment also reversed many transcriptomic changes associated with HFpEF but had no effect on cardiac hypertrophy.
Using a similar rat model of HFpEF, Cho et al. investigated the potential effects of CDC therapy on ventricular arrythmias.37 At 4 weeks after the intracoronary infusion, CDC therapy was associated with shortening of action potential duration and increased action potential duration homogeneity. CDC-treated animals were also less prone to ventricular arrhythmia induction by programmed electrical stimulation. Interestingly, CDC therapy was also associated with a regression of diastolic dysfunction as demonstrated by a decrease in E/e’ ratio and a decrease in left atrial size.
Based on the results of these two studies, there is a positive signal that cell therapy may improve some parameters of diastolic function in HFpEF. Nevertheless, there is a clear need for additional studies to verify and expand these preliminary findings.
Clinical Evidence for Cell Therapy in HFpEF
To date, our group has performed several clinical trials investigating the effects of CD34+ cell therapy in patients with HFrEF. We also evaluated the effects of this approach on diastolic parameters in a group of patients with non-ischaemic dilated cardiomyopathy.38 We enrolled 38 dilated cardiomyopathy patients with New York Heart Association class III and left ventricular ejection fraction (LVEF) <40% who underwent transendocardial CD34+ cell transplantation. Peripheral blood CD34+ cells were mobilised by granulocyte-colony stimulating factor, collected via apheresis, and injected transendocardially in the areas of myocardial hibernation. Patients were followed for 1 year. At baseline, estimated filling pressures were significantly elevated (E/e’ ≥15) in 18 patients (Group A), and moderately elevated (E/e’ <15) in 20 patients (Group B). During follow-up there was an improvement in diastolic parameters in Group A (E/e’ from 24.3 ± 12.1 to 16.3 ± 8.0; p=0.005), but not in Group B (E/e’ from 10.2 ± 3.7 to 13.2 ± 9.1; p=0.19). Accordingly, in Group A, we found an increase in 6-minute walk distance (from 463 ± 83 m to 546 ± 91 m; p=0.03), and a decrease in N-terminal pro B-type natriuretic peptide (NT-proBNP) from 2140 ± 1743 pg/ml to 863 ± 836 pg/ml (p=0.02).
Based on the results of this trial we next aimed to perform a pilot clinical study of CD34+ therapy in patients with HFpEF.39 In a prospective crossover study, we enrolled 30 patients with HFpEF (LVEF >50%, E/e’ >15, NTproBNP >300 pg/ml). In Phase 1, patients were treated with medical therapy for 6 months. Thereafter, all patients underwent transendocardial CD34+ cell transplantation. They received bone marrow stimulation with filgrastim (10 μg/kg, 5 days); CD34+ cells were collected by apheresis. We performed electroanatomical mapping of the left ventricle and injected the cells transendocardially in the areas of diastolic dysfunction. Patients were followed for 6 months after the procedure (Phase 2). In Phase 1, we found no change in E/e’ (from 18.0 ± 3.5 to 17.4 ± 3.0; p=0.97), global systolic strain (from −11.5 ± 2.4% to −12.8 ± 2.6%; p=0.17), NT-proBNP levels (from 1463 ± 1247 pg/ml to 1298 ± 931 pg/ml; p=0.31), or 6-minute walk test distance (from 391 ± 75 m to 402 ± 93 m; p=0.42). In contrast, in Phase 2, we found a significant improvement in E/e’ (from 17.4 ± 3.0 to 11.9 ± 2.6, p<0.0001), a decrease in NTproBNP levels (from 1298 ± 931 pg/ml to 887 ± 809 pg/ml; p=0.02), and an improvement in 6-minute walk test distance (from 402 ± 93 m to 438 ± 72 m; p=0.02; Figure 1). Although global systolic strain did not change significantly in Phase 2, (from −12.8 ± 2.6% to −13.8 ± 2.7%; p=0.36), we found a significant improvement of local systolic strain in myocardial segments that were targeted with stem cell injections (−3.4 ± 6.8%; p=0.005).
Although these data are encouraging, they should be viewed as preliminary and interpreted with caution, particularly because of the lack of placebo-controlled design. Furthermore, as patients with HFpEF are typically older and present with several comorbidities, the effects of autologous cell therapy in these patient populations may be limited because of decreased cell numbers and impaired cell viability. One approach to improve the therapeutic efficacy of autologous cell therapy in HFpEF may thus be based on strategies to intervene in aspects of the stem cell aging process.40 Alternatively, this limitation could be overcome by the use of allogeneic cell products from healthy donors that could be used as an off-the-shelf therapeutic product. Taking these limitations into account, the results of this trial may serve as a foundation for further, larger trials exploring the potential clinical benefits of cell therapy in patients with HFpEF.
Conclusion
Although complex, the principal underlying mechanisms of HFpEF development and progression appear to be based on endothelial inflammation, leading to microvascular rarefaction and myocardial fibrosis. In various preclinical and clinical settings cell therapies have been consistently associated with anti-inflammatory effects, improved angiogenesis and a decrease in myocardial fibrosis and may thus represent an interesting novel treatment approach for HFpEF. The current preliminary evidence investigating the use of cell therapy in HFpEF shows a positive signal, which should be further validated in future preclinical and clinical studies.