Heart failure (HF) is a pandemic, chronic degenerative disease estimated to affect 38 million people worldwide, a number expected to increase with the ageing of the population.1 As a syndrome, it is associated with high mortality and morbidity, and consistently requires increasing resources. HF is the most frequent cause of hospitalisation in patients aged over 65 years, and hospitalised patients have a much worse prognosis than those who are stable at home.2–5 Although therapies can significantly improve symptoms and clinical condition, mortality and rehospitalisation rates remain as high as 10% within 30 days in Europe and are almost 25% in Medicare beneficiaries in the US. Such readmissions are often considered to be a marker of poor healthcare and have become a benchmark for reimbursement and an indicator of hospital quality.6,7 In spite of investments in this field, reductions in the major adverse events have not been achieved, and the 30 days after discharge is a critical, delicate period.8,9
In this article, we will consider treating patients after hospitalisation for acute HF, focusing on the vulnerable period just after discharge from hospital and on the therapeutic interventions found to reduce readmissions.
Heart Failure Rehospitalisation: An Unsolved Problem
Recent observations from clinical practice have shown length of stay and in-hospital and 30-day mortality for patients with HF have reduced, but the readmission rate at 30 days after hospitalisation for HF is higher.10 While congestion is the primary cause of HF relapse, and subclinical congestion develops days or even weeks before an acute event, only 17–35% of rehospitalisations are due to HF exacerbation; most admissions of people with HF are related to non-cardiovascular (CV) causes, such as renal disorders, arrhythmias, sepsis and pulmonary disease.11
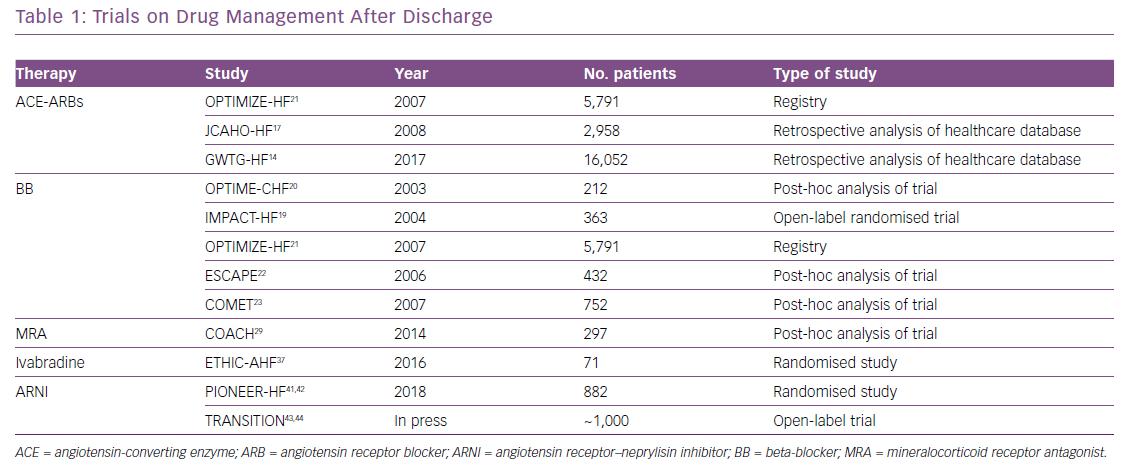
Due to the heterogeneity of readmission triggers, a focus on prevention strategies to reduce CV and non-CV causes of 30-day rehospitalisation are needed, and measures to predict readmission should be highlighted and promoted among both consultant physicians and community care providers. This article provides an overview of HF therapy in the acute phase after hospitalisation. Table 1 shows some of most important clinical trials on HF drugs effect after discharge.
Medical Therapy Following Hospitalisation for Acute Decompensated Heart Failure
International guidelines of the American College of Cardiology and the European Society of Cardiology (ESC) underline the importance of starting and continuing with HF medications, such as angiotensin-converting enzyme inhibitors (ACEIs), angiotensin-receptor blockers (ARBs), beta-blockers (BBs) and mineralocorticoid receptor antagonists (MRAs), during an episode of acute HF and after discharge.12,13 These medications are considered the mainstay of chronic HF therapy, and their use is recommended as class I indication in these patients.
Despite strong evidence for the benefits of these drugs, their use by clinicians is not always routine, especially after an episode of acute decompensated HF. Data from the Get With the Guidelines-Heart Failure (GWTG-HF) registry showed that, of patients already receiving medications for chronic HF at the time of hospitalisation, 88.5% of those taking ACEIs, 91.6% of those on BBs and 71.9 % of those taking MRAs continued to take them from admission through discharge.14 MRAs were the first medications to be discontinued during hospitalisation (in 28% of patients), followed by ACEIs (13%) and BBs (2.6%). A multivariate analysis of predictors of evidence-based medication use at discharge showed that the most significant variables were younger age and taking medications on admission.14
Furthermore, an analysis of the prospective, multicentre, observational ESC-HF Long-Term Registry in Europe showed a significant increase in the rate of prescription of all HF medications at discharge compared to the period before admission.15
Angiotensin-converting Enzyme Inhibitor or Angiotensin Receptor Blocker
Previous studies have shown conflicting results regarding continuation of ACEIs/ARBs during an episode of acute HF. Fonarow et al. analysed data from the Organized Program To Initiate Lifesaving Treatment In Hospitalized Patients With Heart Failure (OPTIMIZE-HF) registry and found that in patients with HF with reduced ejection fraction (HFrEF) the prescription of ACEI/ARBs at discharge was associated with a significant lowering of risk only for the composite endpoint of death and rehospitalisation at 60–90 days, and no difference in overall mortality was observed.16
On the contrary, the analysis of the Joint Commission on Accreditation of Healthcare Organizations Heart Failure (JCAHO-HF) study showed that ACEI/ARB therapy was associated with improved 1-year survival after HF hospitalisation.17 In addition, a recent sub-analysis of the GWTG-HF registry showed that 30-day mortality was 3.5% for patients continuing ACEIs/ARBs, while it was 8.8% for patients discontinuing (p<0.001). Moreover, the 30-day readmission rate was lowest among patients still on therapy at discharge. The same benefits persisted at 1 year (mortality 28.2% for patients continuing on ACEIs/ARBs, compared to 41.6% for patients off therapy; p<0001).18
Beta-blockers
There is only one randomised trial that investigated the effect of pre-discharge carvedilol initiation in patients stabilised after an episode of acute HF, the Initiation Management Predischarge: Process for Assessment of Carvedilol Therapy in Heart Failure (IMPACT-HF) study. The results showed pre-discharge initiation of carvedilol is safe, well tolerated and has a good short-term compliance.19 A retrospective analysis of the Outcomes of the Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) study reported that, of 212 people treated with BB at the time of the admission for decompensated HF, the 47 patients who permanently stopped BB had a worse outcome.20 Results of the OPTIMIZE-HF study registry showed that patients discharged with BBs had a lower risk of death from any cause at 60–90 days than discharged without it (HR 0.46; p<0.006).21 Similarly, in a post-hoc analysis of the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization (ESCAPE) trial, patients discharged on BB therapy had a significantly lower 180-day death or rehospitalisation rates. This association remained significant when data were adjusted for propensity to use BB at discharge and covariates associated with death or rehospitalisation (OR 0.51; 95% CI [0.27–0.97]; p<0.01).22 Moreover, in a post-hoc analysis of the Carvedilol or Metoprolol European Trial (COMET), patients were subdivided into three groups: those who received the same dose before and after HF hospitalisation; those who had a dose reduction of at least one level at the visit following discharge from hospital; and those who were taken off the study drug. The results of the analysis found that 1- and 2-year cumulative mortality rates were significantly higher in patients withdrawn from the study medication or those with a reduced dosage than to those maintained on the same dose, independent of the type of BB used. The result remained significant in a multivariable model (HR 1.30; 95% CI [1.02–1.66]; p=0.0318).23
Mineralocorticoid Receptor Antagonist
Few studies have analysed the effect of MRAs in this clinical scenario. In an observational analysis of 43,625 patients admitted with HF and discharged home, Albert et al. found that only 33% of those who were eligible to be treated with an MRA actually received one.24 Curtis et al. investigated data from the GWTG-HF study linked with Medicare claims to examine adherence and persistence in the use of MRAs among Medicare beneficiaries for whom this therapy had been indicated.25 They observed that only one in five eligible patients was prescribed an MRA at discharge; moreover, eligible patients without a prescription at discharge seldom started therapy as outpatients. All these analyses showed the use of MRAs is extremely low in patients with acute HF, although this medication is strongly recommended, especially in those with advanced HF. Moreover, data on continuation of MRA in Medicare beneficiaries hospitalised for acute HF showed it improved the 60-day survival rate.26 In the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD) study, Hamaguchi et al. investigated the effect of spironolactone on survival and hospitalisation among hospitalised patients with systolic HF.27 They noticed that use of spironolactone was associated with a significant reduction of all-cause death (adjusted HR 0.612; p=0.02) and cardiac death (adjusted HR 0.524; p=0.013), while no effect was found for hospitalisation.
In the Comparative Effectiveness of Therapies for Heart Failure (COMPARE-HF) registry, Hernandez et al. analysed the clinical effectiveness of newly initiated aldosterone antagonist therapy among older patients hospitalised with HFrEF. The result of the study showed that the use of aldosterone was not associated with reduced risk of mortality at 3 years after discharge (p=0.32), even though readmissions for HF were lower among treated patients at 3 years (p=0.02).28 The use of aldosterone was associated with higher risk of hospitalisation at 30 days and 1 year due to hyperkalemia. In addition, results from the Co-ordinating Study Evaluating Outcome of Advising and Counselling in Heart Failure (COACH) biomarker study showed that patients who remained on spironolactone treatment had a lower 30-day mortality.29
Diuretics
Diuretics are considered only a symptomatic therapy in patients with chronic HF, since their effects are mostly aimed at reducing congestion and they have no impact on survival and on rehospitalisation. One small, randomised, open-label study examined the differences on clinical outcomes between furosemide and torsemide in patients admitted to the hospital for an episode of acute HF.30 The results suggested that patients treated with torsemide were less likely to be readmitted for HF and for all CV causes versus those taking furosemide.
An analysis from the Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan (EVEREST) trial showed that patients responsive to diuretic therapy and those with haemoconcentration were both at lower risk for early post-discharge adverse events.31 Moreover, an analysis from the Diuretic Strategies in Patients with Acute Decompensated Heart Failure (DOSE-AHF) study reinforced these results, showing improved clinical outcomes at 60 days for patients with adequate loss of weight, fluid removal and natriuretic peptide reduction after treatment during HF hospitalisation.32
Digoxin
Digoxin has been extensively evaluated by the Digitalis Investigation Group (DIG) trial, which shows it reduces all-cause and HF hospitalisations.33 However, its role and effect in acute HF was investigated only in a registry based on the Alabama Heart Failure Project, in which 8,049 patients hospitalised with a primary diagnosis of HF were observed for 30 days after discharge. In this study, digoxin seemed effective in reducing 30-day, all-cause readmission only in patients with a left ventricular ejection fraction (LVEF) <45% (HR 0.63; 95% CI [0.47–0.83].34
Ivabradine
In a small cohort of patients admitted for acute HFrEF with a heart rate (HR) >70 BPM and no need for inotropic treatment, ivabradine significantly reduced HR and was associated with improved NYHA class and N-terminal pro-brain natriuretic peptide (NT-proBNP) levels.35 Similar results were obtained in a retrospective analysis on 29 patients.36 In addition, the effects of ivabradine and BBs were compared to BBs alone in a randomised trial including 71 patients with acute HFrEF and sinus rhythm with HR >70 BPM. HR at 1 and 4 months after discharge was significantly lower in the beta-blockers plus ivabradine group, and there were significant improvements in LVEF and natriuretic peptides, but no differences in clinical events at 4 months.37 Therefore, it has been suggested that ivabradine could be given in addition to BB therapy to improve HR control in patients with acute HF.38 However, evidence is still sparse, and the safety and efficacy of ivabradine needs to be confirmed by other clinical trials.
Angiotensin Receptor–Neprilysin Inhibitors in the Transition Phase
Angiotensin receptor–neprilysin inibithors (ARNIs) are a novel HF treatment. This innovative therapy has been investigated in a multicentre prospective randomised study, the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure (PARADIGM-HF) trial, which compared sacubitril/valsartan with enalapril in patients with chronic HF, reduced ejection fracture (EF; <40%), and a New York Heart Association (NYHA) II–IV classification.39 The primary results of this study showed a significant decrease in both cardiovascular mortality and HF hospitalisation with ARNIs with respect to enalapril. The study was interrupted prematurely at 27 months of follow-up because the primary endpoint occurred in 914 patients (21.8%) in the sacubitril/valsartan group versus 1,117 patients (26.5%) in the enalapril group, reflecting a 20% reduction in the composite of CV death or HF hospitalisation in the former. In addition, sacubitril/valsartan reduced both the time to first hospitalisation for HF, and the cumulative burden of HF hospitalisation. Based on these excellent data, the use of ARNIs has gained a class I indication in patients with chronic HFrEF able to tolerate ACEI/ARB therapy in recent ESC guidelines.13
Desai et al. investigated the effect of ARNIs on the rates of all-cause 30-day readmission after an HF hospitalisation.40 They analysed all patients who survived after the first HF admission. A total of 1,450 patients were investigated, including 675 (16.1%) assigned to sacubitril/valsartan and 775 (18.4%) assigned to enalapril. The results showed that the use of ARNIs had a significant effect on reducing the rate of readmission for any cause or for HF at 30 and 60 days.
The safety and efficacy of sacubitril/valsartan compared to enalapril among patients hospitalised for acute HF were investigated in a randomised study, Comparison of sacubitril/ valsartaN versus Enalapril on Effect on NT-pRoBNP in patients stabilised from an acute Heart Failure episode (PIONEER-HF). The results showed no differences in renal dysfunction, hyperkalemia, symptomatic hypotension and angioedema between the two treatment arms. Noteworthy, the reduction in the NT-proBNP concentration was significantly greater in the sacubitril/valsartan group than in the enalapril group.41 In summary, sacubitril/valsartan was recognised to be more effective than enalapril among stabilised patients hospitalised for acute HF in reducing natriuretic peptides and the composite of rehospitalisation for HF or CV death.42
Based on these promising results, the Pre-discharge and posT-discharge tReatment initiation with sacubitril/valsartan in heArt failure patieNtS with reduced ejectIon fracTion hospItalised for an acute decOmpensation eveNt (TRANSITION) study aimed to investigate the effects and tolerability of ARNIs in patients stabilised after hospitalisation for acute HF, regardless of whether they received it while in hospital or after discharge.43 TRANSITION involved 1,002 randomised patients, of whom 287 (29%) had new-onset HF with reduced EF, and 243 (24%) were ACEI/ARB naive. Pre-admission use of BBs and MRAs was lower than in PARADIGM-HF; moreover, TRANSITION patients were older, more likely to be female and have worse renal function, and a higher proportion of them had AF and diabetes. At 10 weeks after randomisation, 45% of patients in the pre-discharge arm and 50.4% of patients in the post-discharge arm achieved the target dose of 200 mg sacubitril/valsartan twice daily (relative risk ratio [RRR] 0.893; 95% CI [0.783–1.019]; p=0.092). More than 85% of patients achieved and maintained any dose of sacubitril/valsartan for at least 2 weeks leading to week 10 after randomisation in both groups (86.4% of those who started it before discharge initiation and 88.8% of those who began it after discharge; RRR 0.973; 95% CI [0.929–1.020]; p=0.262). Mortality rates were low in both treatment arms (p=0.258) and none of the deaths was attributed to the study treatment. Therefore, the TRANSITION preliminary results demonstrated the safety and tolerability of starting sacubitril/valsartan in stabilised HFrEF patients shortly after an acute HF event.44
Medical Therapy Following Hospitalisation for Acute Decompensated Heart Failure in Advanced Heart Failure
Continuous and intermittent infusion of intravenous inotropes have been used in different clinical scenarios of advanced HF and end-stage HF (eHF).45,46 Different inotropes have been investigated, but most of these trials were based at one centre, so enrolled a limited number of patients.47 Moreover, the majority of these studies have several statistical limitations, such as being a retrospective analysis or not using a randomised, placebo-control methodology.
Levosimendan is a calcium sensitiser and potassium channel opener with inotrope and vasodilatory effects, and it is the most promising inotrope tested in advanced HF trials.48 Among these, only three studies have investigated its role in reducing acute HF hospitalisations: the Intermittent Intravenous Levosimendan in Ambulatory Advanced Chronic Heart Failure Patients (LION–HEART) study, the Long-Term Intermittent Administration of Levosimendan in Patients With Advanced Heart Failure (LAICA) study and the Relevant-HF REpetitive LEVosimendan in AdvaNced refracTory Heart Failure (RELEVANT-HF) registry.49–51 In the LION-HEART study, 69 patients were randomised to either levosimendan (n=48) or placebo (n=21) administered in an ambulatory setting. The primary endpoint was change in NT-proBNP from baseline, and the secondary endpoint was a reduction in the combined incidence of all-cause mortality and hospitalisation. Both were significant in favour of levosimendan, in particular hospitalisations for HF, at 22.9% versus 66.7% in the placebo group (HR 0.25; 95% CI [0.11–0.55]; p=0.001).
In the LAICA study, 97 patients (levosimendan, n=70; placebo, n=27) were randomly assigned to receive infusions once every 30 days in addition to optimal standard HF therapy.50 The primary endpoint was the incidence of admission for advanced HF or HF worsening. No significant differences were observed, but there were fewer admissions for advanced HF and lower mortality rates in the levosimendan group (6.6% versus 22.2% for placebo; p=0.0439).
In the RELEVANT-HF registry, 185 ambulatory patients were treated in a hospital or outpatient setting with specifically tailored intermittent levosimendan therapy.51 The study showed a significant reduction in the number and duration of HF-related hospitalisations and in total days in hospital.
Based on the results of these studies, repetitive infusion of levosimendan in advanced HF seems promising and an effective way to reduce HF hospitalisations.52
Unsolved Problem: Drug Underutilisation After Discharge
Obstacles limiting the correct, early prescription of an optimised medical therapy for acute HF at discharge are mainly related to drug adverse effects in patients who are still fragile. The introduction and maintenance of an optimal medical treatment may be challenging, as the time available to test the drugs may be short, and the patient may have several comorbidities preventing a correct dosage of the drug from being used.
Potential factors that could lead a clinician to underutilise or discontinue HF drugs include symptomatic hypotension, worsening renal function, angioedema, electrolyte disturbance and a period of tissue hypoperfusion. The ESC guidelines recommend patients admitted to hospital for acute HF should receive evidence-based, oral medication for at least 24 hours before discharge to reduce the possibility of drug discontinuation.13 Nonetheless, randomised trials substantiating a benefit for starting the patient on medication before versus after discharge are lacking. Data from the GREAT registry, including 19,980 patients with acute HF with both reduced and preserved ejection fraction HFpEF, showed that BBs and ACEIs/ARBs at discharge led to a reduction in 90-day mortality, and had a better impact on overall long-term survival.53
Interestingly, only in patients with HFpEF was a positive association found between oral MRA at discharge and 90-day mortality. It may be that the failure of MRA to show a beneficial effect in patients with HFrEF could be related to a higher rate discontinuation because of hyperkalemia, which is a common adverse effect, especially in elderly patients taking diuretics. As comorbidities are typical in patients with HFpEF, a tailored approach to treat both cardiac and non-cardiac comorbidities could help physicians in maintaining a good uptitration of HF drug, as treating these includes controlling blood pressure, monitoring heart rate and heart rhythm, lowering glycaemic and lipid profiles, and favouring a healthy lifestyle.
Multidisciplinary Disease Management Program After Discharge
To ensure better drug adherence after HF hospitalisation, a multidisciplinary disease management programme should be established and encouraged on a large scale. The key elements of a multidisciplinary programme include hospital HF physicians, specialised HF nurses, a well-structured network between primary care and tertiary centres, and regional HF outpatient clinics. Usually, specialised HF nurses are responsible for programme coordination, which involves home visits, optimising treatment, early recognition of worsening HF and facilitating patient empowerment.54 Such programmes, consequently, improve patient wellbeing, reduce hospitalisations and increase overall survival rate.
Conclusion
Rehospitalisation after an acute HF event is one of the main issues affecting patients’ short- and long-term prognosis. The first 30 days after discharge are a delicate period, where both cardiologists and community care providers should work together to reduce exacerbation of the disease. A proper use of HF drugs during hospitalisation and just after discharge should be promoted and emphasised by international guidelines to improve HF management and patient quality of life.