Natriuretic peptides (NPs) are well established in the diagnostic process of heart failure (HF). Low levels of NPs are particularly useful to exclude heart failure (HF). Numerous studies, predominantly in patients presenting with acute onset of symptoms suspected of HF, have convincingly shown the value of NPs in this regard. A meta-analysis published in 2015 clearly summarised the value of brain (B-type) NP (BNP), N-terminal proBNP (NT-proBNP) and mid-regional pro-atrial NP (MR-proANP) in the acute setting, uniformly showing a very high negative predictive value when using low cut-off levels (i.e. BNP <100 pg/ml; NT-proBNP <300 pg/ml, MR-proANP <120 pmol/l).1
These cut-off values, recommended by the European Society of Cardiology (ESC) guidelines, have an excellent ability to exclude acute HF, missing only a few cases. They help to distinguish HF from non-cardiac causes of dyspnoea.2,3 However, the specificity is modest and variable, indicating that confirmatory diagnostic testing by cardiac imaging is required if the result is positive. In addition, the negative predictive value varies quite significantly between studies if higher cut-off values are used.1
For patients in the outpatient setting not presenting with acute symptoms, the recommended cut-off values are lower, at 35 pg/ml for BNP and 125 pg/ml for NT-proBNP.2 Levels above these values are also required as part of the diagnosis of patients with HF and preserved ejection fraction (HFpEF; LVEF ≥50%) or mildly reduced (mid-range; HFmrEF; LVEF 40–49%) left-ventricular ejection fraction (LVEF).2 No recommendation is given for MR-proANP as no larger studies in the outpatient setting have been published.
Outpatient cut-off values are less investigated than cut-off values in the acute setting, particularly with respect to BNP. The best cut-off values found in these studies are not as uniform as various guidelines may recommend; only some are in this range, with many of them being higher and more in the range of the values recommended in the acute setting, as well as some being lower.4–9
In addition, the negative predictive value may be less than it is in the acute setting, which possibly relates to diagnostic accuracy reducing with increasing age (i.e. c-statistics decreasing from 0.95 in patients aged <50 years to 0.82 in patients aged >75 years), as shown in a meta-analysis including >5,500 patients from 10 studies to test the diagnostic value of NT-proBNP to detect LVEF≤40%.10 These authors suggested using an age-specific cut-off value to rule out HF in primary care settings, which would be lower than recommendations in the current US and ESC guidelines for young (<50 years, cut-off value 50 pg/ml) and middle-aged people (50–75 years, 75 pg/ml), but higher for elderly patients (>75 years, 250 pg/ml).10 Still, this analysis has not been considered by the guidelines and the suggested cut-off values differ between European, US and UK guidelines.2,11,12
While the majority of these studies did not distinguish between HF with reduced ejection fraction (HFrEF; LVEF <40%) and HFpEF, some studies focused on diastolic dysfunction only.13 Although NP levels are lower in HFpEF in general, the established thresholds for diagnosing acute HF remain useful in patients with preserved ejection fraction, with only minor loss of diagnostic performance (NPV 90% at a BNP of 100 pg/ml).14 The distinction may be less relevant in the acute setting, where therapy is largely similar regardless of LVEF. In chronic HF however, treatment of chronic HFrEF is well defined, in contrast to that for HFpEF.2 In addition and especially in the elderly, NPs have a much poorer diagnostic performance for HFpEF.15
Another study showed the added value of NT-proBNP in diagnosing HF, with increasing levels being added to a score including nine clinical features (age, coronary artery disease, loop diuretics use, pulse rate and regularity, displaced apex beat, rales, heart murmur and elevated jugular vein pressure) to diagnose HF.16 This score had high c-statistics of 0.86 in the derivation set and higher c-statistics of 0.88 and 0.95 in two external validation sets.16 The problems with such a score are that it is not easily applicable in clinics and there is a significant zone of uncertainty.
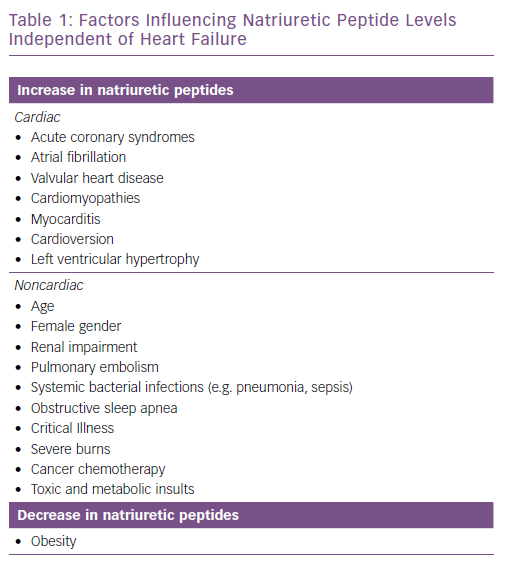
A recent meta-analysis investigated cut-off values using point-of-care devices in both the acute and ambulatory outpatient settings.17 This analysis may provide the currently most accurate overview of the diagnostic accuracy of NPs in the ambulatory setting. Data from primary care were scarce and ranges of cut-off values varied widely, particularly for BNP. The ESC recommended cut-off level for the non-acute setting (35 pg/ml) was not used in any of the included studies, whereas the value for NT-proBNP (125 pg/ml) was used in four studies.17 However, results depend on the patient population included and may vary, depending on the prevalence of HF. Additional studies are needed to identify the best cut-off values in the non-acute setting to diagnose HF. In addition, many factors independent of HF may influence NP levels (Table 1).
Although there is an obvious overlap of the cardiac causes and HF itself, interpretation of NP levels in individual patients must be done against the background of additional factors influencing these values. For example, in a young patient with no comorbidities, expected NP levels are very low, whereas in elderly patients with reduced renal function and atrial fibrillation levels clearly above the cut-off value are common even in the absence of HF.
Recent data support the application of different thresholds of NT-proBNP for the diagnosis of HFpEF in patients with AF versus those in sinus rhythm.18 Importantly, obese patients with HF can have normal values of NPs, even if they are volume overloaded; the Breathing-Not-Properly study found that the best cut-off value in severely obese subjects is much lower than in lean patients.19 However, prospective validation of these types of individualised cut-offs is lacking and so is not yet supported by HF guidelines.
Measurement of NPs may also help as screening tool in primary care to stratify patients and reduce risk. The Irish Screening To Prevent Heart Failure (STOP-HF) study randomised patients at risk for developing HF into either usual care or additional measurement of BNP levels.20 If BNP was 50 pg/ml or higher, patients were referred for echocardiography. This resulted in more cardiovascular investigations and more treatment, but less HF and left ventricular dysfunction.20 The measurement of BNP in this setting was likely to be cost effective.21 Although these results are promising, confirmation in other populations and healthcare systems is still absent.
The finding that intensifying medical therapy may result in less chronic HF in high-risk patients with elevated NP levels is supported by the Austrian NT-proBNP Selected PreventiOn of cardiac eveNts in a populaTion of dIabetic patients without A history of Cardiac disease (PONTIAC) trial in people with diabetes with NTproBNP levels >125 pg/ml but free from any cardiac diseases.22 MR-proANP has not been studied in this regard but was shown to stratify risk for the development of cardiovascular mortality and incident HF in patients with coronary artery disease. In addition, only patients with at least two of three biomarkers elevated – MR-proANP, MR-proADM and CT-proET-1 – showed an improvement in outcome with ACE-inhibition.23 Obviously, this is no proof that interventions based on MR-proANP levels would result in better outcome, which needs to be prospectively investigated.
Taken together, BNP and NT-proBNP levels are diagnostically useful not only in the acute setting but also in the diagnostic process of chronic HF and possibly in the identification of patients at risk of developing HF. Still, more research is needed in the ambulatory setting. Therefore, cut-off values to exclude HF are not yet clearly defined and their diagnostic value might be less than in the acute setting. In addition, the value of MR-proANP has not yet been tested in this setting. Confirmatory studies are required to define the role of NPs in identifying patients at risk who need advance diagnostics and more aggressive medical therapy.
Prognostic Value of Natriuretic Peptides in Chronic Heart Failure
Without doubt, NPs are strong prognostic markers in patients with chronic HF. This is true for all NPs for which tests are commercially available. However, although NPs may be considered as the most robust prognostic markers in chronic HF, individual studies indicate some variety, showing some other biomarkers having a better prognostic value. Despite this, no single biomarker is clearly prognostically superior to NPs. In many instances, other biomarkers representing different pathophysiological pathways provide additional prognostic information to NPs.24 A 2005 systematic review showed the prognostic value of BNP, including identifying changes over time, in a large number of studies and this number has increased considerably since then.25It is beyond the scope of this review to discuss these studies and recent reviews discussing the prognostic value of NPs in detail.26,27
More important are the clinical consequences of knowing the prognosis of an individual patient. Guidelines recommend risk assessment by using risk scores to inform management decisions on advanced therapy such as ventricular assist devices and cardiac transplantation, despite no studies showing the clinical value of this recommendation. One may argue that patients at high risk should be monitored more closely. To the best of the authors’ knowledge, it has not yet been investigated whether basing frequency of consultations on prognostic markers results in better outcomes and if such an approach would be cost-effective. However, more specialised treatment with scheduled follow-ups does not seem to improve outcome as the NorthStar trial shows.28 Also, the number of visits per se also does not seem to influence outcome.29
Nevertheless, NPs are increasingly used in clinical trials, based on their prognostic value to better predict event rate and to increase the study power by including patients at higher risk. A prominent recent example is the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial, which investigated the effect of sacubitril/valsartan compared to enalapril in chronic HFrEF.30 To meet the inclusion criteria, patients had to have a BNP level of ≥150 pg/ml or NT-proBNP level of ≥600 pg/ml or 100 pg/ml and 400 pg/ml, respectively, if hospitalised within the previous 12 months. Such inclusion criteria based on NPs are not the result of specific underlying pathophysiology but related purely to the strong prognostic value of NPs. In addition, NPs may predict sudden cardiac death and, therefore, might be helpful for indication of ICD implantation.31 However, such an approach needs to be prospectively tested before NPs can be recommended as selection criterion. Combining the strong prognostic value of NPs, together with the fact that NP levels change with altered therapy, make them an interesting guide for therapy in HF.32
Therapy guidance using natriuretic peptides in chronic heart failure
Due to the prognostic power of NPs and because many patients with HF do not meet the target doses of HF drugs as recommended by guidelines, many studies have been conducted to test the hypothesis that therapy guided by repeated measurements of NPs improves outcome compared to usual care.
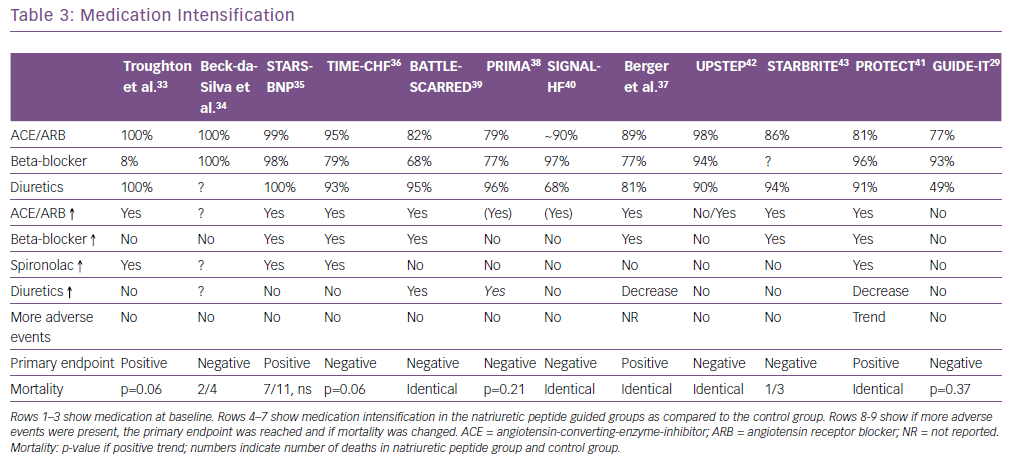
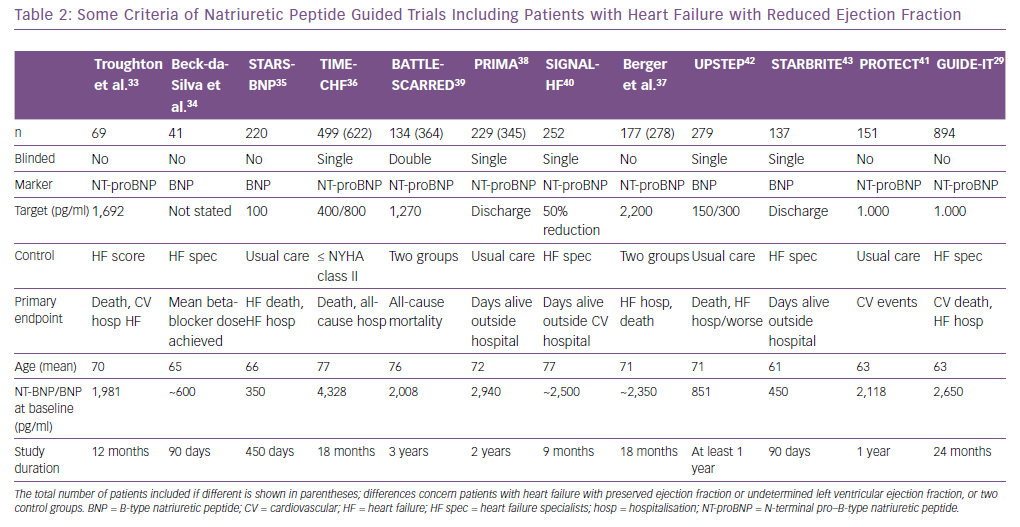
The first study investigating this hypothesis was published in 2000 but, 19 years and more than a dozen trials later, it has not been established whether this hypothesis is true or not.33 This is in part related to the fact that none of the NP-guided trials was large enough to convincingly show the effects of this approach. This may be true even for the GUIDE-IT trial, which is the latest and largest study investigating this topic, which was stopped early so did not meet the predefined sample size and follow-up.29 Even more importantly, there is a large variation between the trials29, 33–43 with regard to several aspects, including that: the included populations differed significantly; the interventions were not uniform; and follow-up length and the number of time-points to adjust therapy varied (Tables 2 and 3).
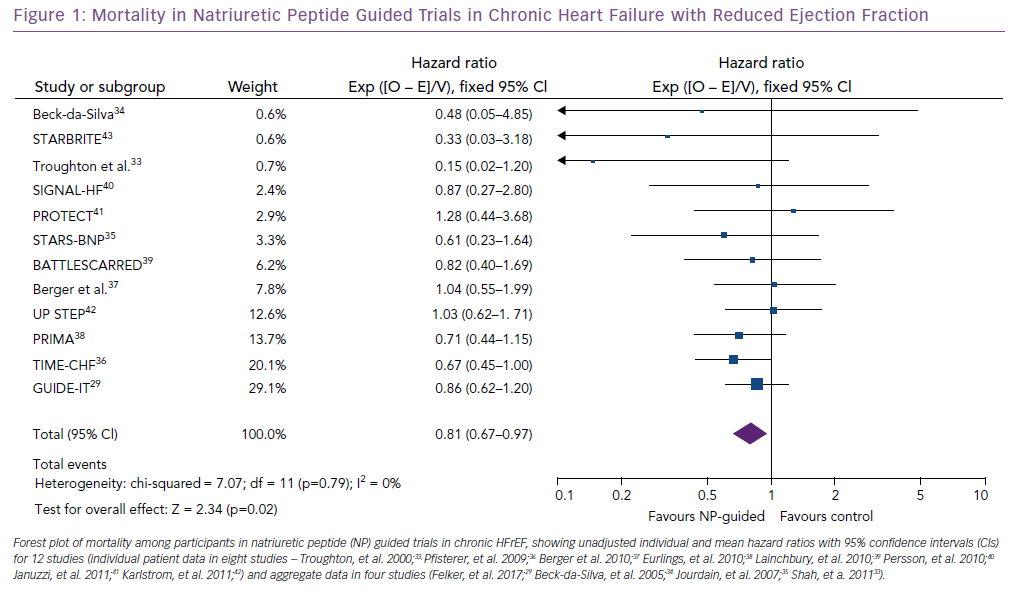
A recent meta-analysis came to conclusion that NP-guided therapy does not result in any benefit.44 However, this meta-analysis did not properly account for the large diversity between the trials, nor did it perform sufficient sensitivity analyses despite including different kind of studies that are not directly comparable. Strikingly, the use of NPs both in the acute setting and in chronic HF was combined in this investigation and studies were included regardless of whether they included patients with HFrEF, HFpEF or both. It is well known that HFpEF does not respond to classic HF therapy, and a previous meta-analysis based on individual patient data showed a different response to NP-guided therapy in HFrEF and HFpEF.2,45 In addition, one study (NorthStar) included in this meta-analysis suggested action should be taken only if NT-proBNP levels significantly increased but not if they remained elevated and it included both HFrEF and HFpEF. Not surprisingly, adjustments in therapy were limited and identical in the two treatment arms and, consequently, NT-proBNP hardly changed.46 Most other NP-guided trials showed a significant reduction in NP levels in both treatment arms (e.g. Felker, et al, 2017; Pfisterer, et al, 2009).29, 36 The only genuinely relevant group of patients in whom NP guidance in chronic HF should be investigated are those with HFrEF. When only results in chronic HFrEF from the previous trials (1,507 in the NP-guided group and 1,516 in the control group) are included, NP-guided therapy – mostly using NT-proBNP, some using BNP – resulted in a significant reduction in mortality (Figure 1).29,33–43 Overall, 222 (14.7%) patients died in the NP-guided group and 275 (18.1%) in the control group.
As far as we can say, NP-guided therapy seems to be safe, even in elderly patients with significant comorbidities, and may be cost-effective as well.47–49 However, further evidence from more trials on NP-guided therapy is required.
Trials where adjustment in therapy did not differ between the NP-guided versus the clinical guided group, or where increasing loop diuretics was the main difference, usually showed a neutral outcome. In contrast, trials where the focus was mainly on intensifying guideline-recommended medication (e.g. angiotensin-converting enzyme [ACE] inhibition or angiotensin-receptor blockers, beta-blockers or mineralocorticoid receptor antagonists) showed some positive results although, because of a lack of statistical power, the primary endpoint of the trials was not always reached (Table 3). This shows that applying guidelines when managing patients with HFrEF is crucial to improve outcome.
The fact that NP guidance in TIME-CHF resulted in significantly larger uptitration of evidence-based treatment even in patients aged >75 years indicates that, in many patients with HFrEF, the maximum tolerated has not been reached and forced uptitration is feasible.36 Unfortunately, this is often not done in reality and the question arises over what may encourage physicians to do more for these patients. Measuring NPs to indicate the importance of uptitration may help in this regard.
Sacubitril: A Problem for Measuring Natriuretic Peptides?
Among the bioactive peptides, sacubitril reduces the breakdown of the biologically active NPs by inhibiting the enzyme neprilysin, a circulating neutral endopeptidase involved in the degradation of NPs.50 This is true for both ANP and BNP, but BNP is a poorer substrate for neprilysin than ANP. Therefore, the increase of BNP may be less.51 The increase in BNP was only small although significant whereas the increase in urinary cGMP was much larger with sacubitril/valsartan in the PARADIGM-HF study.52
Sacubitril has no direct influence on NT-proBNP because neprilysin has no effect on cleavage of NT-proBNP. However, it might be speculated that an increasing level of BNP results in a negative feedback regarding production of proBNP, thereby reducing NT-proBNP. To the best of our knowledge, this has not been properly tested. In clinical trials, sacubitril/valsartan resulted in a reduction of NT-proBNP, as well as a small increase in BNP as mentioned above.52,53 This reduction was accompanied by a better outcome. Therefore, it is likely that the decrease in NT-proBNP is, at least in part, related to more effective treatment of HF. To what extent NT-proBNP levels are influenced by a negative feedback mechanism remains to be determined.
With the more widespread clinical use of sacubitril/valsartan, the measurement of serum NP levels in patients taking this drug may change and a rethinking of their interpretation is required. In patients taking sacubitril/valsartan, levels of BNP may rise because of decreased serum breakdown rather than because of a change in underlying disease state (such as volume overload in AHF), which potentially interferes with the prognostic and diagnostic utility of BNP.27 However, this does not impair the clinical utility of BNP testing to rule out HF rapidly. Also, the clinical interpretation of NT-proBNP levels in patients with HF is probably not affected in a clinically meaningful way by neprilysin inhibition, based on published data.
When to Measure Natriuretic Peptides in Chronic Heart Failure
First, NPs are useful in the initial diagnosis or exclusion of HF. They may also help to identify patients at risk of developing HF where early intervention may reduce risk.
Second, NPs should be used in the outpatient management of HFrEF when deciding whether to start a patient on eplerenone (BNP >250 pg/ml or NT-proBNP >500 pg/ml in men or >750 pg/ml in women, unless they have been hospitalised within the previous 6 months because of HF) or sacubitril/valsartan (BNP >150 pg/ml or NT-proBNP >600 pg/ml; in case of a HF, hospitalisation within the previous 12 months, BNP >100 pg/ml or NT-proBNP >400 pg/ml), in line with the ESC HF guidelines, which are based on the inclusion criteria of the respective drug trials.2
Third, despite a lack of sufficient evidence for the superiority of natriuretic guided-therapy overall, NPs can help to decide whether a patient is being treated optimally. This is supported by the American College of Cardiology/American Heart Association guidelines (class IIA level of evidence), although this recommendation might change after the recent GUIDE-IT trial, and is not mentioned in the ESC HF guideline.2,11 Based on (pre-stratified) subgroup analyses, we would recommend this approach mainly in patients who have HFrEF and few comorbidities.45 Based on trials, it seems best to use a target around normal values, meaning ~125 pg/ml for BNP and ~1,000 pg/ml for NT-proBNP (Table 2).
The focus should be on improving and intensifying drugs that improve outcomes, such as renin–angiotensin system blockers, beta-blockers and mineralocorticoid receptor antagonists in patients whose (NT-pro)BNP levels remain elevated, not on the use of intensified diuretic therapy. This could be done merely to convince patients or their caregivers that (further) uptitration of evidence-based medicine is crucial.
As to whether a lack of elevated NPs means that further uptitration of medication is not required remains to be prospectively tested in a randomised trial. This may be relevant in patients who are most susceptible of side effects (e.g. frail elderly people).
Fourth, NPs may help to distinguish whether an increase in symptoms is related to worsening HF or deterioration of another condition (e.g. chronic obstructive pulmonary disease). When patients are using sacubitril/valsartan, the preferred NP is definitely NT-proBNP.