The National Institutes of Health Biomarkers Definitions Working Group define a biomarker as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention”.1 Biomarkers have become increasingly important in current medical practice as they offer an easy way to either diagnose an illness or to monitor progress. Tijsen et al. have suggested that an ideal biomarker ought to be easy to collect non-invasively, should have a high degree of sensitivity and specificity, should be cheap, easily reproducible and should have a rapid measurement system that assists in prompt clinical management.2
For patients presenting with breathlessness, there is a need for a reliable biomarker for the early diagnosis of heart failure. Previous studies have demonstrated a high degree of uncertainty when patients present with breathlessness.3 Heart failure and chronic obstructive airway disease often coexist in approximately 30% of patients, making diagnosis confusing. The Breathing Not Properly study reported clinical confusion in approximately half of cases presenting to the emergency department with breathlessness.4 Echocardiography can detect abnormal left ventricular (LV) function, but that may not be the cause of breathlessness because almost 50% of the community-dwelling population with decreased LV function have been shown to be asymptomatic.5 Hence there is a need for a biomarker that could assist in diagnosis.
Similarly, there is also a need for better monitoring of patients receiving treatment for heart failure. It has been demonstrated that physiological changes often precede clinical deterioration that would lead to a patient attending hospital.6 Invasive mechanisms such as pacemaker devices with physiological monitoring mechanisms can alert the physician to clinical deterioration.7 However, these are invasive and not all patients with heart failure have a pacemaker. Non-invasive means such as a biomarker have therefore become useful.
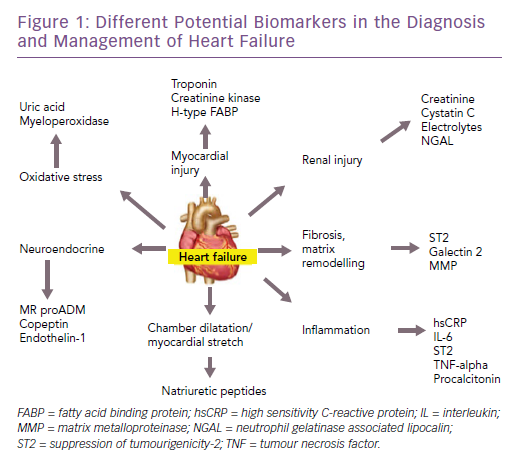
There are many potential biomarkers for heart failure (Figure 1). In this article, we discuss the biomarkers that are available for clinical use in patients with heart failure – both for diagnosis and prognosis – reviewing the evidence and the recommendations of various guidelines. Furthermore, we will highlight some of the emerging biomarkers in this field, along with the evidence for their use.
Biomarkers for Diagnosis
The diagnosis of heart failure in a patient presenting with breathlessness for the first time is often difficult, and biomarkers – along with other investigations – can contribute to diagnosis. Traditionally, clinical presentation along with chest X-ray has been used to make a diagnosis of heart failure. However, studies have repeatedly shown a low sensitivity and specificity for making a clinical diagnosis of heart failure. Echocardiography is a useful component of diagnosis, but in the acute setting it may not always be possible to obtain an echocardiogram, particularly out of hours. Additionally, the echocardiogram may be normal in heart failure with preserved ejection fraction (HFpEF).
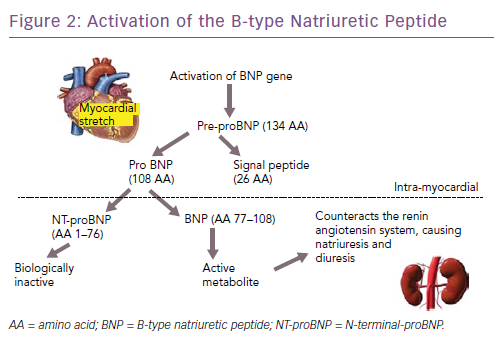
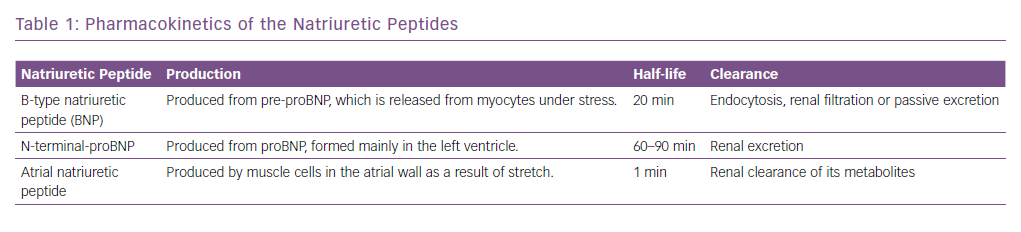
The natriuretic peptides are the most extensively studied and used biomarkers in heart failure.8 As a result of myocardial stretch, the B-type natriuretic peptide (BNP) gene is activated and prohormone proBNP1–108 is produced. This is cleaved to the biologically active BNP and the biologically inert but stable NT-proBNP1–76. They down-regulate the sympathetic system, cause diuresis, decrease peripheral resistance and increase smooth muscle relaxation (Figure 2). Atrial natriuretic peptide (ANP) as rapid clearance and is less consistent as a diagnostic marker and hence is not used routinely. However, newer assays have been developed that measure the precursor hormone of ANP, mid-regional proANP (MR-proANP). MR-proANP is more stable, giving more reliable results, and has therefore been identified as a reliable marker. The pharmacokinetics of these molecules is shown in Table 1.9
The Breathing Not Properly Study was one of the first major trials studying the role of natriuretic peptides in the emergency department for the diagnosis of heart failure.10 Here the authors measured BNP levels in 1,586 patients presenting to the emergency department with acute breathlessness. Patients with clinically diagnosed heart failure had higher BNP levels compared with those without heart failure (mean 675 ± 450 pg/ml versus 110 ± 225 pg/ml; p=0.001). Increasing severity of heart failure, as measured by New York Heart Association (NYHA) functional class, correlated directly with increasing concentrations of BNP (p<0.001). BNP was the best single predictor of a final diagnosis of heart failure compared with all individual history, physical examination, chest x-ray and laboratory findings. A cut-off BNP value of 100 pg/ml had a sensitivity of 90% and a specificity of 76%. In addition, BNP was more accurate (83%) than either the National Health and Nutrition Examination Survey criteria (67%) or the Framingham criteria (73%), two established criteria for heart failure diagnosis. Importantly, the best method of diagnosis of heart failure was seen when BNP and clinical findings were combined.
The use of NT-proBNP in the diagnosis of acutely decompensated heart failure was first demonstrated in the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) study.11 Here, NT-proBNP had a high sensitivity for the diagnosis of heart failure, again supplementing clinical judgment as BNP did in the Breathing Not Properly study. Subsequently, the International Collaborative Of NT-proBNP (ICON) study examined optimal applications of NT-proBNP in 1256 acutely dyspnoeic patients.12 Patients with acutely decompensated heart failure had considerably higher NT-proBNP concentrations compared with those without heart failure (4,639 pg/ml versus 108 pg/ml; p<0.001) and symptom severity correlated with NT-proBNP concentrations (p=0.008). As natriuretic peptide concentrations rise with increasing age, the ICON investigators found the best approach for use of NT-proBNP in heart failure diagnosis was through use of age-stratified cut-off points; this approach improved the positive predictive value of the assay considerably.
The utility of MR-proANP in the diagnosis of heart failure was demonstrated in the Biomarkers In Acute Heart Failure (BACH) study.13 In the diagnosis of acute heart failure in those presenting to the emergency department with dyspnoea, a MR-proANP level greater than the predefined cut point of 120 pmol/l was found to be non-inferior to BNP at the 100 pg/ml cut point. Combining MR-proANP and BNP increased diagnostic accuracy from 73.6% with BNP alone to 76.6%. It was also found that in cases where BNP and NT-proBNP could be less informative (obesity, old age, renal dysfunction or ‘grey zone’ values), MR-proANP added value when used in combination with each biomarker. Thus it has been suggested that the addition of MR-proANP with other natriuretic peptides adds to diagnostic accuracy.
It should be remembered that there are many other causes of raised natriuretic peptides besides heart failure.14 These include cardiac causes such as acute coronary syndrome, myocarditis, cardioversion etc., along with non-cardiac causes such as age, anaemia and renal failure. Conversely, obesity has been shown to decrease natriuretic peptide levels.14
Kim and Januzzi have suggested cut-off points for different scenarios.14 For BNP, they have suggested a ‘grey zone’ approach. A value of <100 pg/ml would exclude heart failure and >400 pg/ml would confirm heart failure. For those in the ‘grey zone’ of 100–400 pg/ml, further tests would be required. For NT proBNP, an age-stratified approach is suggested. Values <450 pg/ml would be used as a cut-off for patients aged <50 years, <900 pg/ml for those aged 50–75 years and <1,800 pg/ml for those aged >75 years. In patients with renal dysfunction, (glomerular filtration rate <60 ml/min/1.73 m2), a BNP cut-off value of 200 pg/ml or NT-proBNP of <1,200 pg/ml should be used. Similarly, different cut-off values for BNP have been suggested based on BMI. A cut-off of 170 pg/ml is recommended for BMI <25 kg/m2, 110 pg/ml for BMI 25–35 kg/m2 and 54 pg/ml for BMI >35 kg/m2. No correction is required for NT-proBNP based on BMI. All these values have a high sensitivity and specificity.
Among the other non-natriuretic-peptide biomarkers, the troponins are often elevated in patients with heart failure.15 However they only represent myocardial injury and are therefore not specific for making a diagnosis of heart failure. They could also be increased in any condition that puts increased stress on the heart muscle. They may also be useful in diagnosing concomitant acute coronary syndromes in the presence of heart failure.16
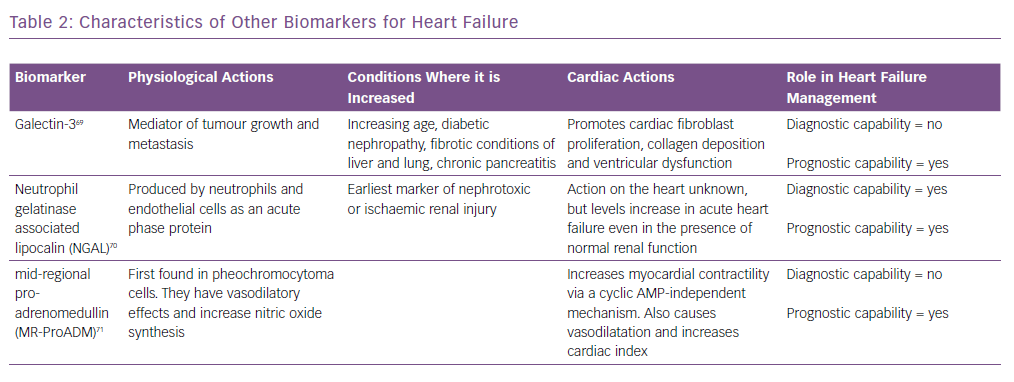
Similarly, biomarkers, such as soluble suppression of tumourigenicity-2 (ST2), galectin-3 and pro-adrenomedullin, are also increased in patients with heart failure.17 However, they are not useful for the diagnosis of heart failure as they are not specific for these patients and are increased in other conditions as well.18 Their characteristics are summarised in Table 2 and are discussed in detail in the prognosis section.
The American and European guidelines on the management of heart failure both give measuring natriuretic peptides for the diagnosis of heart failure a class 1A recommendation.19,20 The European guidelines recommended that the upper limit of normal in a non-acute setting is 35 pg/ml for BNP and 125 pg/ml for NT-proBNP. In the acute setting, the cut-off values are higher at 100 pg/ml for BNP and 300 pg/ml for NT-proBNP. At these cut-off values the negative predictive values are similar and high at 0.94–0.98 in both the acute and non-acute settings but the positive predictive values are low. Therefore it has been suggested that the use of the natriuretic peptides are mainly for ruling out a diagnosis of heart failure rather than establishing it, when there is clinical uncertainty. However, at higher natriuretic peptide values, the positive predictive value is high. The American guidelines do not specify any cut-off values. Both sets of guidelines mention that other biomarkers are elevated in acute or stable heart failure, but they do not recommend their routine use for the diagnosis of heart failure.
Biomarkers for Prognosis
The natriuretic peptides again are the most extensively investigated biomarker for assessing prognosis of patients with heart failure – both in the acute setting as well as for patients with chronic heart failure seen in the office setting. It has been shown that at baseline, the higher the BNP, the worse the prognosis, with patients having almost a five-fold greater mortality between the highest and lowest tertiles.21
In patients admitted with heart failure, the risk of readmission and death is high if the discharge BNP is not lower than the admission value.22 Many of the large heart failure studies have also examined the role of biomarkers in prognosis. In the Valsartan Heart Failure Trial (Val-HEFT), patients with the greatest fall in BNP with treatment had the best prognosis.3 Similarly, in the Organized Program To Initiate Lifesaving Treatment In Hospitalized Patients With Heart Failure (OPTIMIZE-HF) study, discharge BNP was shown to affect prognosis.24 A meta analysis by Doust et al. found that for every 100 pg/ml increase in BNP there was a 35% increase in the risk of death.25
In the Framingham study, it was shown that even in asymptomatic patients without overt heart failure, every standard deviation of the log BNP value was associated with a 27% increase in the risk of death, 28% increase in first cardiovascular event, 77% increase in the risk of heart failure, 66% increase in AF and a 53% increase in stroke/transient ischaemic attack.26 However there was no relation with coronary artery events. Similar results were also obtained from community-dwelling populations in the Omsted county study.27
In a comparison of NT-proBNP and MR-proANP using a sample of 525 chronic heart failure patients of all NYHA classes, MR-proANP was found to be positively correlated with NYHA class, and – after correction for NT-proBNP, age, ejection fraction, NYHA class, creatinine, and BMI – MR-proANP was found to be a predictor of poor survival.28 In the PRIDE study, elevated MR-proANP was independently prognostic and reclassified mortality risk at 1 year (HR 2.00; p<0.001) and at 4 years (HR 3.12; p=0.001).29 MR-proANP was also associated with death up to 4 years, both alone and with other biomarkers. In chronic heart failure, the Gruppo Italiano Perlo Stuio Della Sopravvivenza Nell’insufficienza Cardiaca Heart Failure (GISSI-HF) study,30 showed that the prognostic accuracy for MR-proANP for mortality was best with an area under the curve (AUC) of 0.74 (95% CI [0.71–0.77]) with an optimal cut-off point of 278 pmol/l, followed by NT-proBNP with an AUC of 0.73 (95% CI [0.70–0.76]) and an optimal cut-off of 1,181 pg/mol. Changes in MR-proANP over 3 months also appeared to be predictive of future mortality.
Among the other non-natriuretic-peptide biomarkers, high baseline troponin corresponded to a worse prognosis with an OR of 2.5 for death within a year.31 Serial measurements of high sensitivity troponins (hsTn) during a hospitalisation for acute heart failure can risk stratify patients for 90-day mortality and readmission.15 It has been shown that patients whose discharge troponin value rose compared with the admission value had the greatest risk.32 Another study showed that an elevated hsTn as well as a >20% increase in the value was associated with increased mortality.33 The prognostic value is enhanced when combined with natriuretic peptides.34 Here the troponins are likely to reflect the level of myocardial strain and stress secondary to the heart failure rather than a coexisting acute coronary syndrome.
Adrenomedullin (ADM) is a 52-amino acid peptide thought to be upregulated as a result of increased volume overload and is mediated by vasoactive hormones. However, because of its rapid clearance from the circulation and short half-life (22 minutes), using ADM as a routine biomarker is impractical. MR-proADM, the mid-regional segment of ADM’s precursor pre-proADM, is released in equimolar concentrations as ADM and thus is an effective substitute, and because of its inactivity and longer half-life, MR-proADM is a better surrogate marker. The BACH trial13 ADM appeared to predict 90-day mortality or rehospitalisation due to cardiovascular causes better than BNP/proBNP. Similar results were also reported by Klip et al.35 ADM was also found to be predictive of mortality in a cohort of community-dwelling patients.36
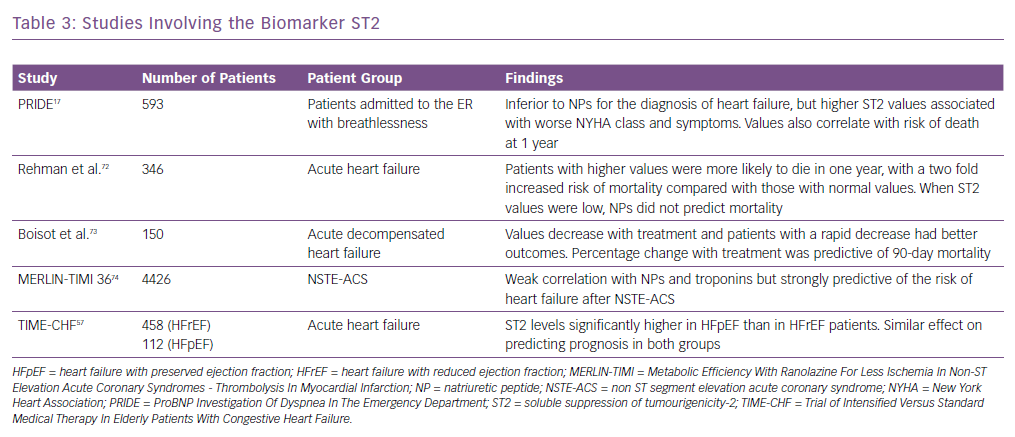
Other biomarkers, including ST2, have been shown to be associated with adverse outcomes in heart failure and predict mortality risk in these patients. It is also known as interleukin-1 receptor-like 1, and is a member of the interleukin-1 receptor family.37 In the PRIDE study17 ST2 values >0.20 ng/ml had an increased risk of death at 1 year. It was better than other biomarkers in both acute and chronic heart failure in predicting prognosis and works synergistically with the natriuretic peptides to enhance mortality prediction in acute and chronic heart failure. Similarly, in the Val-HEFT study, change in ST2 values over time was significantly and independently associated with mortality.38 It has also been shown to be predictive of mortality and cardiovascular events in non-ischaemic dilated cardiomyopathy.39 Some of the important trials highlighting the usefulness of ST2 are summarised in Table 3.
Galectin-3 is secreted by activated macrophages and causes cardiac fibrosis by proliferation of cardiac fibroblasts.40 It also regulates inflammation, immunity and cancer, and can act as a surrogate marker of cardiac remodelling and the fibrosis that is seen in heart failure. It has not been shown to be useful in diagnosis, but has strong prognostic value. In the Pravastatin Or Atorvastatin Evaluation And Infection Therapy – Thrombolysis In MI 22 (PROVE-IT-TIMI 22) study,41 higher galectin-3 levels correlated with the development of heart failure. Similarly, in the Coordinating Study Evaluating Outcomes Of Advising And Counselling In Heart Failure (COACH) trial,higher levels increased the risk of death or rehospitalisation over 18 months.42 Its value also correlated with inflammatory markers such as C-reactive protein, vascular endothelial growth factor and interleukin-6. It has also been shown to predict mortality in non-ischaemic dilated cardiomyopathy.39,43
Numerous studies however have shown that when more than one biomarker is studied, they predict prognosis much better than the individual markers alone. For example, Gaggin et al. demonstrated that a model that contains clinical data, NT-proBNP, hsTn1 and ST2 along with endothelin-1, had a very good predictive value.44 This is understandable because each of these markers studies the impact of heart failure on various different pathophysiological processes that comprise heart failure.
The American heart failure guidelines recommend the use of natriuretic peptides and troponins for risk stratification and for determining prognosis in both acute and ambulatory patients with heart failure.19 The European guidelines mention the role of biomarkers in determining prognosis, but do not issue any specific recommendations.20
Biomarkers as a Guide For Therapy
Studies have consistently shown that patients whose BNP or NT-proBNP values show greater reductions tend to have better prognosis.23 It would therefore appear logical that we could use BNP values to guide therapy with frequent monitoring of the values to assess whether patients need more intense heart failure treatment.45 However, results have been conflicting and not entirely as expected.
Early studies were promising. In the Systolic Heart Failure Treatment Supported By BNP (STARS-BNP) trial,Jourdain et al. randomised 220 patients with NYHA functional class II and III to either routine medical therapy or to a natriuretic-peptide-guided therapy where the aim was to reduce BNP to <100 pg/ml.46 At 15 months, there were far fewer clinical end points (heart-failure-related death or hospitalisation) in the BNP-guided group (24% versus 52%; p<0.001). However those in the BNP-guided arm had significantly higher physician visits and drug changes although only around a third of patients reached the target BNP value of <100 pg/ml. Similarly the Pro-BNP Outpatient Tailored CHF Therapy (PROTECT) trial by Januzzi et al. with 151 subjects also showed a benefit for patients who had NT-proBNP-guided therapy for heart failure.47
Despite the initial positive trials, later larger trials were not so convincing. The NT-proBNP–Assisted Treatment To Lessen Serial Cardiac Readmissions and Death (BATTLESCARRED) trial randomised 364 patients with heart failure to either natriuretic-peptide-guided therapy, clinical-guided therapy or usual care.48 They found that intensive heart failure management that was guided by NT-proBNP monitoring was associated with improved mortality compared with usual care. However, when compared to clinical guided therapy, natriuretic-peptide-guided therapy improved long term mortality only in patients aged <75 years.
The Trial of Intensified versus Standard Medical Therapy In Elderly Patients With Congestive Heart Failure (TIME-CHF) randomised trial on the other hand did not find any benefit either in terms of quality of life or cardiovascular outcomes with intensive management guided by NT-proBNP.49 Similarly, the Can Pro-Brain-Natriuretic Peptide Guided Therapy Of Chronic Heart Failure Improve Heart Failure Morbidity And Mortality? (PRIMA) study also failed to show any benefit with natriuretic-peptide-guided therapy.50
Troughton et al. performed an individual patient data meta-analysis of the various trials that studied the effect of natriuretic peptide monitoring during heart failure therapy.51 They identified 11 eligible studies, of which eight had individual patient data (n=2,000). Pooling the data, they found that there was a survival benefit in the group that had natriuretic peptide monitoring. However, when classified according to age, this benefit was seen only in those aged <75 years and not in those >75 years of age. The authors explain that perhaps in the elderly, due to intolerance, optimal drug dosages would not have been achieved and hence explain why monitoring natriuretic peptide values did not improve mortality. The superior mortality benefit in the younger group could conversely be explained by the fact that these patients tolerated the higher dosages of the drugs and were able to achieve maximal dosages of guideline-directed medical therapeutic agents. The meta-analysis also noted significant benefit in terms of hospital readmission rates in those where treatment was guided by natriuretic peptide monitoring.
Similarly, a recent Cochrane review of the subject concluded that there was low-quality evidence to suggest that natriuretic peptide-guided therapy could lead to a reduction in heart failure admissions, but there was uncertainty regarding the effect of natriuretic-peptide-guided therapy on mortality and all cause admission and quality of life.52
Other biomarkers such as ST2 have also been shown to change with therapy.53 The use of beta-blockers and mineralocorticoid receptor blockers have been shown to reduce elevated ST2 levels. However, data are lacking in large trials studying specifically the utility of other markers besides the natriuretic peptides in guiding therapy.
The American guidelines give a Class IIa (level of evidence B) recommendation for the use of BNP or NT-pro BNP to achieve optimal dosing for guideline-directed medical therapy in select euvolaemic patients (in the outpatient setting) who are followed up in a well-structured heart failure management programme.19 However, they suggest that using serial natriuretic peptide monitoring during therapy does not help in reducing hospitalisation or mortality in either the ambulatory outpatient setting or in the acute decompensated setting.
The European guidelines do not advocate the use of natriuretic peptides in monitoring the progress of patients being treated for heart failure, stating there is insufficient data to recommend it.20
Heart Failure with Preserved Ejection Fraction
Most of the studies of biomarkers in heart failure are confined to patients with heart failure with reduced ejection fraction (HFrEF). This could be due to the fact that HFpEF has been defined as a separate and distinct entity much more recently compared with the traditional HFrEF subgroup, and also because HFpEF is generally more difficult to diagnose clinically. Studies have shown that the natriuretic peptides are moderately increased in HFpEF and that values fall to normal during symptom-free periods.54,55 Although the sensitivity of these biomarkers is slightly lower for patients with HFpEF compared with HFrEF, it still has a high diagnostic accuracy.
Markers of inflammation such as ST2 have been shown to be increased in HFpEF patients and correlated well with pro-inflammatory comorbidities.56 In a study of 458 patients with HFrEF and 112 patients with HFpEF, ST2, high sensitivity C-reactive protein and cystatin C levels have been shown to be higher in HFpEF than HFrEF, while NT-ProBNP and troponin values were higher in HFrEF.57 However, although they predicted prognosis to a similar level in both types, Manzano-Fernandez et al. showed that ST2 values were lower in HfpEF than HFrEF, while maintaining their prognostic predictability.58
Similarly, markers of myocardial fibrosis like galectin-3 have been shown to be elevated in HFpEF. In the COACH study, higher levels of galectin-3 were associated with higher rates of rehospitalisation and death in HFpEF but not HFrEF patients.42 Despite this, studies have failed to show any correlation between levels of galectin-3 and measures of cardiac structure and function including left ventricular geometry.59
The role of biomarkers in the diagnosis of HFpEF has recently been reviewed by Michalska-Kasiczak et al.60 They conclude that one single biomarker may not be sufficient for the correct diagnosis of HFpEF as it is a very heterogeneous group of patients. They suggest that a panel of biomarkers including mRNAs may be required.
Because of the paucity of data, neither the American nor the European guidelines differentiate between the two subgroups with regards to the biomarkers.19,20
Newer Biomarkers and Future Prospects
Many new biomarkers that have been studied in heart failure. However most of these have limited data and often fall short when compared to the NPs. These biomarkers target different aspects of the pathogenesis of heart failure, such as myocardial injury, inflammatory response, renal injury and volume status. Some of the novel ones, for example ST2, galectin 3 and pro-ADM, have been discussed earlier.
Neutrophil gelatinase-associated lipocalin is expressed by neutrophils and epithelial cells.61 It is a marker of renal injury. The values are also high in heart failure, even when the reductions in renal function or minimal. Studies such as Optimal Trial In Myocardial Infarction With The Angiotensin II Antagonist Losartan (OPTIMAAL)and NGAL Evaluation Along With B-type Natriuretic Peptide (BNP) In Acutely Decompensated Heart Failure (GALLANT) have demonstrated a role for this marker in the diagnosis and prognostic prediction in patients with heart failure.62,63
Another exciting prospect is the role of circulating microRNA (miRNA) in heart failure. It has been shown that these are differentially expressed in the failing heart.64 Different miRNAs, such as miR423-5p, miR320a and miR22, have been shown to be increased in patients with heart failure.65 A recent meta-analysis of the role of miRNAs in the management of heart failure suggested that miR423-5p offered the best potential as a biomarker.66 However, large-scale trials are required to validate their utility.
Many other molecules, such as procalcitonin, matrix metalloproteinases, interleukins and tumour necrosis factor alpha, have been studied in heart failure. However none of them are specific and have variable findings. It is most likely that future heart failure biomarker studies would involve a panel of markers, including natriuretic peptides, ST2 and hsTn1, which study the different pathophysiological processes that are involved in heart failure. The use of genetic testing including miRNA could become more widespread.
Metabolomic profiling (study of the byproducts of metabolism) and transcriptomics (the study of complete sets of RNA transcripts produced by the genome) are another two areas that are undergoing extensive research in the field of heart failure. Initial studies have been promising but more research is required to see if these become standard of care for patients with heart failure in the future.67,68
Conclusion
The use of biomarkers in the management of patients with heart failure has increased tremendously over the past few years. Currently the natriuretic peptides are the most commonly used biomarker and help in the diagnosis and prognostication of patients with heart failure. Their role in the monitoring of treatment is still debatable, although it seems reasonable that patients have their natriuretic peptide values checked at discharge.
There are many new biomarkers currently under investigation. The results are promising and they evaluate different aspects of the heart failure spectrum. At present they appear to have a synergistic role along with the natriuretic peptides – both in terms of diagnosis and determination of prognosis. However, on their own, none of them are specific for heart failure and none are recommended for routine clinical use at present. Further research is required to see which of the newer agents can be used as a reliable biomarker for the diagnosis and monitoring of patients with heart failure.