With this letter, the authors have the pleasure to present their views to the Editor-in-Chief. Based on our experience, we would like to discuss isolated left ventricular (LV) apical hypoplasia.
In 2006, a 64-year-old Caucasian woman presented to the Emergency Department with breathlessness. She was an ex-smoker with a medical history of hypertension, hypercholesterolaemia and asthma. There was no family history of cardiac disease. Initial investigations, including chest radiograph, showed pulmonary oedema, which was treated with IV diuretics and continuous positive airway pressure non-invasive ventilation.
ECG performed on admission showed left bundle branch block (LBBB). An echocardiogram showed severe LV systolic dysfunction with global hypokinesis (Supplementary Material Video 1). A coronary angiogram showed normal coronary arteries and a ventriculogram suggested severe LV impairment, apical akinesis, aneurysmal dilatation of the inferior wall and a possible mural thrombus. Her condition improved with medical therapy and the patient was discharged on furosemide, perindopril, bisoprolol and anticoagulation for the suspected mural thrombus.
At outpatient clinic follow up, she was stable with New York Heart Association (NYHA) class II symptoms and discharged from routine follow-up.
She remained stable in the community for 11 years until June 2017, when she re-presented to cardiology with worsening breathlessness and NYHA class III symptoms. ECG showed AF with an LBBB, QRS duration of 140 ms and a ventricular rate of 76 BPM. An echocardiogram showed a severely dilated LV with severe LV impairment (Supplementary Material Video 2). Her heart failure medication was optimised with the addition of eplerenone 25 mg daily.
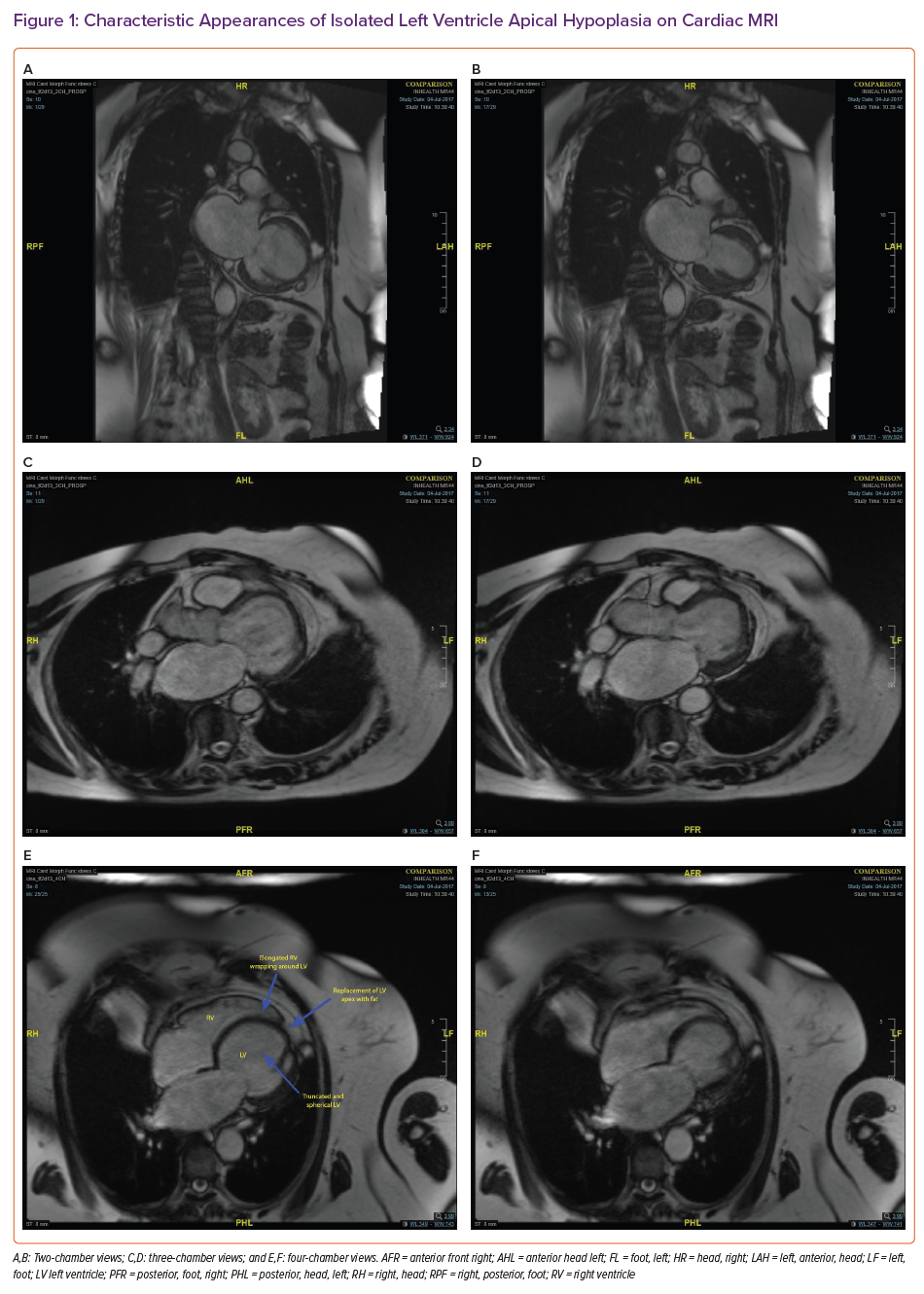
In contrast to 2006, the cardiology service in 2017 had access to cardiac MRI (CMR) to investigate the aetiology of the LV dysfunction; this showed absence of the LV apex with a spherical, truncated LV and bulging of the interventricular septum towards the right ventricle (RV). There was replacement of the LV apex with fat contiguous to the epicardial fat and the LV appeared moderately impaired with a reported ejection fraction of 45%. The RV was elongated at the apex and wrapped around the deficient LV apex. There was abnormal apical origin of the papillary muscle network. There was no significant late gadolinium enhancement seen (Figure 1 and Supplementary Material Videos 3, 4 and 5).
A diagnosis of isolated LV apical hypoplasia was made. The patient’s case was discussed in the device multidisciplinary team meeting and a decision for CRT pacemaker (CRT-P) implantation was made. Although the implantation of a CRT-P led to no significant improvement in effort tolerance, the patient continues to remain stable, has not had any further admissions for decompensated heart failure and has reached the age of 79 years. More recently, she developed difficulty with rate control of AF, resulting in decreased biventricular pacing. Medical therapy, including the addition of amiodarone, was attempted with no beneficial impact on effort tolerance and with unacceptable side-effects for the patient, leading to the need to stop the medication. The patient therefore had atrioventricular nodal ablation for poor rate control, which has improved the biventricular pacing rate and symptoms.
Discussion
Isolated LV apical hypoplasia is a rare condition that was first described in 2004 by Fernandez-Valls et al., who used CMR and cardiac multi-detector CT to show characteristic morphological changes of what appeared to be a previously undiscovered LV congenital abnormality in three patients.1 These patients varied in age from 22 to 46 years, two of whom were women, and none had a relevant family history. They all had an otherwise negative cardiomyopathy screen.
Since then, there have been very few cases reported in the literature and very little knowledge currently exists regarding what appears to be a rare congenital disease. The cause is unknown but may be related to inadequate LV–RV dilatation during partitioning, which could lead to a spherical LV and an elongated RV that wraps around the LV apex. A genetic defect may be involved and there has been one case reported in which a mutation of the lamin A/C gene was identified, a gene known to be associated with other forms of cardiomyopathy.2,3 There is currently an ongoing clinical trial with the aim of looking for a genetic basis of the condition by performing genetic testing with whole exome sequencing in seven patients with isolated LV apical hypoplasia (NCT04339582).
It is important to distinguish the condition from other congenital conditions. For example, hypoplastic left heart syndrome occurs in 3% of infants with congenital heart disease, and although there is reduction in size in the LV cavity, there are also associated features such as atresia or stenosis of the mitral valve, aortic valve or aorta, which the present patient did not have. Furthermore, hypoplastic left heart syndrome is fatal in the first few weeks of life without intervention.4
Isolated LV apical hypoplasia has a variable phenotype with presentations ranging from asymptomatic to congestive cardiac failure, as seen in the present patient.5–7 A case series of five young patients with the condition describes cyanosis in two of the patients, although these patients had associated persistent ductus arteriosus and severe pulmonary hypertension.8 Indeed, there has been a case report of death on first presentation in a 19-year-old who died of ventricular tachyarrhythmias and multi-organ dysfunction.9
Definitive diagnostic criteria have not yet been established.1,10 The characteristic features on CMR are:
- truncated, spherical and impaired LV with bulging of the interventricular septum towards the RV;
- apical LV fatty material;
- papillary muscle/trabecular abnormalities; and
- RV elongation wrapping around the deficient LV apex.
In the present patient there was a discrepancy in the assessment of LV systolic function using 2D transthoracic echocardiography and CMR. The 2D echocardiographic assessment of LV function is often based on Simpson’s biplane method, which has limitations if the apex is foreshortened or if there is endocardial dropout. This method may not be accurate in the assessment of systolic function of a spherical LV with apical hypoplasia. CMR is a volumetric technique and has a high contrast and spatial resolution, leading to improved accuracy and reproducibility in the assessment of the LV.11 It is the modality of choice in the assessment of LV apical hypoplasia given that it has the benefit of not depending on assumed geometry.12
However, it is noted that there have been reports of characteristic findings of the condition being seen on echocardiography leading to a correct diagnosis.13,14 Indeed, in retrospect, the present patient’s echocardiogram in 2006 and 2017 did suggest features of LV apical hypoplasia but these were not fully appreciated due to a lack of awareness of the condition.
There is little known about the management or prognosis of LV apical hypoplasia given the relatively low number of cases that have so far been identified. Some cases reported in the literature have responded to recommended first-line heart failure therapy.1 The present patient did respond to these heart failure treatments and remained stable for 11 years before further presentation with heart failure. Our management plan following deterioration involved further up-titration of her heart failure medications and cardiac device therapy.
The patient had an LBBB with a QRS duration of 140 ms, impression of severe LV systolic dysfunction on echocardiogram and deteriorating effort tolerance with NYHA class III symptoms. Therefore, a decision was made to proceed with CRT-P despite the higher ejection fraction measured on cardiac MRI. The trials on biventricular pacing are based on echocardiography and not cardiac MRI. A biventricular pacemaker with defibrillator (CRT-D) was not implanted, given that there is very little information on the risk of ventricular tachyarrhythmias in isolated LV apical hypoplasia and there was no significant fibrosis on cardiac MRI. The patient has not had any malignant ventricular tachyarrhythmias since implantation of the CRT-P.
This piece describes an interesting case of isolated LV apical hypoplasia in a 79-year-old patient with no family history of cardiovascular disease and two middle-aged daughters with no cardiac disorders. There needs to be increased awareness of this condition to improve diagnostic rates. There were missed early opportunities for diagnosis due to the lack of information about the condition and the low use of cardiac MRI in 2006. The learning points from this case include the importance of continued follow-up and optimisation of heart failure medications and the use of optimised complex cardiac device therapy. Further research is needed to understand the genetic basis of the condition, establish diagnostic criteria and also to understand the natural history of the disease, the risk of tachyarrhythmia and the role of medical and device therapy.