Despite advances in the management of heart failure (HF), a significant burden of mortality and morbidity remains.1 This, combined with the ever-escalating costs of novel drug development, has led to an increased focus on the treatment of comorbidities in order to improve outcomes. As a chronic condition, it is increasingly recognised that HF is actually an iron-deficient state, and is highly prevalent, similar to patients with chronic kidney disease and chronic rheumatological conditions in whom iron therapy is an established part of management. In this review article, we discuss the epidemiology and pathophysiology of iron deficiency (ID) in HF and the current evidence for its utility.
Iron Metabolism
Iron is an important micronutrient that is required by every cell in the body for metabolism. It has a number of important roles that contribute to metabolic health. First, iron is able to transfer between the ferrous (Fe2+) and ferric (Fe3+) states, allowing it to act as a catalyst for important biochemical reactions.2 Iron is a component of haemoglobin and thus plays a key role in tissue oxygenation. It is also a component of myoglobin, which is an oxygen-binding protein found in skeletal muscle and myocytes, allowing oxygen release in hypoxic conditions.3 In addition to being a constituent of haemoglobin, iron plays a key role in erythropoiesis, via hepcidin, which is produced in the liver and regulates iron absorption in the gastrointestinal tract and iron release from reticuloendothelial tissue. ID leads to a reduction in maturation of haematopoietic cells and resistance to erythropoietin, a renally produced cytokine that increases red blood cell development.4 Iron is also a key component of mitochondria, and hence is vital for myocyte energy production. Given these key functions, it is clear to see why targeting ID is an attractive proposition.
It is important to note that total body iron is regulated within a narrow therapeutic window and iron overload, such as in haemochromatosis, can be deleterious, causing cardiac and liver toxicity, as well as oxidative stress.5 The average oral intake of iron is around 10–20 mg/day, and around 1–2 mg is absorbed in the duodenum. There is no pathway for iron excretion; however, around 1–2 mg/day is lost through other mechanisms, such as skin desquamation and bleeding.6 Once absorbed, intracellular iron exists in the ferrous form (Fe2+), while extracellular iron is in the ferric form (Fe3+). Iron is described as either stored (as ferritin within the liver, bone marrow and spleen) or utilised (circulating and intracellular iron).7 Circulating iron is bound to transferrin, which delivers iron to tissues for utilisation or storage, while most intracellular iron is within haemoglobin.
Pathophysiology and Epidemiology of Iron Deficiency in Heart Failure
The prevalence of ID in HF patients has been reported as being up to 50 %, even in patients without anaemia.8–10 The pathophysiology of ID with HF specifically is likely to be multifactorial. When ID is found in patients with HF, it is important not to overlook other causes, such as gastrointestinal ulceration or malignancy. The prevalence of these conditions in patients with ID means that gastrointestinal investigation is often required as first-line to exclude sinister causes.11 There may be simple factors, such as blood loss due to antiplatelet or anticoagulant therapy, leading to iron loss. Importantly though, the use of these medications has not been shown to be correlated with reduced ferritin in HF patients, suggesting that this is not the primary cause of ID.8,9 Malabsorption (and consequent reduced nutrition) may also play a role. A reduction in appetite from chronic illness may lead to a reduction in dietary iron intake. Additionally, gut interstitial oedema can lead to a poorly functioning gastrointestinal tract and reduced oral iron uptake. Liver congestion may also play a role. The chronic inflammatory state associated with HF leads to increased levels of pro-inflammatory cytokines such as interleukin-6 (IL-6). Inflammation induces the synthesis of hepcidin, which leads to reduction in the release of stored iron.12 Intriguingly, while most chronic inflammatory diseases are associated with higher levels of hepcidin, studies in HF patients have actually shown that worse HF is associated with lower levels of hepcidin and does not appear to correlate with IL-6 in this group of patients.13,14 This may be in part due to elevated levels of erythropoietin associated with worse HF and is associated with the suppression of hepicidin.15
In a study of explanted hearts, iron stores within the hearts of HF patients due for cardiac transplant were found to be depleted compared to healthy controls.16 Furthermore, a reduction in soluble transferrin receptor was found in response to increased levels of aldosterone and noradrenaline, which are commonly elevated in HF.
Diagnosis of Iron Deficiency
It is important to note that many HF patients have ID without being anaemic, hence it is vital to screen for ID, even in patients with haemoglobin within the normal laboratory range. The gold standard for the assessment of total body iron stores and diagnosis of ID is bone marrow aspiration with Prussian blue staining. However, this is clearly not practical in the routine setting, particularly if repeated testing is required. The use of serum markers is much more common. Typically, two parameters are used in the assessment of ID: ferritin and transferrin saturation.
Although ferritin is a predominantly intracellular storage molecule, some is able to enter the systemic circulation and can therefore be measured. In stable patients, serum ferritin has been shown to correlate well with overall iron stores measured by bone marrow aspirate.17,18 Traditionally, the normal range for serum ferritin is 30–300 µg/l; however, ferritin is an acute-phase protein and therefore is increased in inflammatory states. For this reason, in most HF studies and in the most recent European Society of Cardiology HF guidelines, serum ferritin <100 µg/l has been used to diagnose absolute ID.19 Absolute ID relates to a state where there is truly insufficient iron. Transferrin levels are usually low. However, low transferrin levels are not necessary for a diagnosis of ID in those in whom serum ferritin is <100 µg/l. Functional ID is defined as a serum ferritin level of 100–300 µg/l and a transferrin saturation of <20 %. In this setting, while the available iron stores are potentially sufficient to meet the body’s physiological demands, they cannot be transported from the intracellular compartment to the circulation. Transferrin saturations are reduced in inflammatory states but they are much less affected than serum ferritin.20
Serum iron levels can also be measured. Levels vary widely, even from hour to hour, so it is recommended that serum iron levels should not be used for the assessment of ID.21 A recent study by Grote Beverborg et al. compared a variety of iron-associated biomarkers against the gold standard of bone marrow biopsy in 42 patients with HF and found that transferrin saturation ≤19.8 % or serum iron ≤13 µmol/l actually had better diagnostic performance than the current definition using serum ferritin, and also had independent prognostic significance in in 387 outpatients with HF.22 One test that has recently been proposed for the diagnosis of ID is serum-soluble transferrin receptor (ssTR) level, which is not affected by inflammation and appears to correlate very well with iron status, being more sensitive but less specific than serum ferritin (although ferritin cut-offs varied within the meta-analysis from which these data were derived).23,24 Despite showing promise, this test is not widely available. There has not yet been any definite incremental benefit to using ssTR above traditional measures of iron status, hence at present serum ferritin and transferrin saturations remain the most useful diagnostic tests.
Consequences of Iron Deficiency
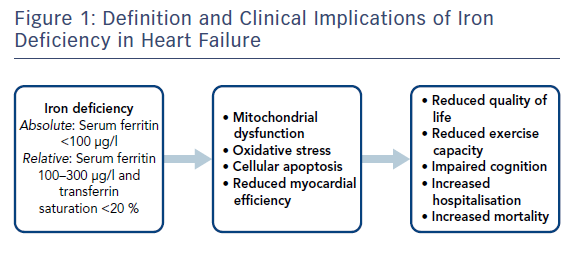
The presence of ID in HF has been shown to have a number of clinical implications. Figure 1 summarises the pathophysiological consequences of ID in HF. ID has been associated with adverse outcomes in several cohorts, including hard outcomes, such as mortality and major adverse cardiovascular events, and softer outcomes, such as quality of life (QoL). In a study of 157 HF patients by Okonko et al., the presence of ID was associated with an over threefold increase in mortality independent of haemoglobin.9 Additionally, non-anaemic patients with ID had double the risk of mortality compared to anaemic patients without ID, suggesting that ID is an independent risk factor in congestive HF patients. These results were replicated in a multicentre international cohort of 1,506 HF patients, with Klip et al. reporting that ID was associated with increased N-terminal pro brain-type natriuretic peptide (NT-proBNP) and worse New York Heart Association (NYHA) class, as well as being an independent predictor of mortality. Several other studies have also shown that ID is an independent predictor of mortality in HF patients.10,25,26
ID has been shown to be independently associated with exercise capacity in HF patients. In a large study of 443 patients with HF who underwent cardiopulmonary exercise testing, patients with ID had lower peak oxygen consumption (VO2 max) and increased ventilator response to exercise compared to those without ID, representing a reduction in exercise capacity.27 When combined with anaemia, the effects seem to be magnified.28 However, absolute ID appears to be independently associated with reduced exercise capacity.29
ID appears to have an impact of QoL in HF patients. Several studies have shown that ID is independently associated with a reduction in QoL, as measured by the Minnesota Living with Heart Failure Questionnaire.30,31
ID has also been shown to affect response to cardiac resynchronisation therapy. In a study 541 patients who underwent cardiac resynchronisation therapy implantation, the prevalence of ID was 56 %. Those with ID had less symptomatic improvement and a lower extent of reverse remodelling than those without ID.32 The presence of ID was also associated with increased mortality and HF hospitalisation independent of the presence of anaemia. One recent small study reported that in addition to a non-left bundle branch block ECG pattern, ID was an independent predictor of response to cardiac resynchronisation therapy in 48 HF patients (defined as reduction in left ventricular end-systolic volume <15 % or increase in VO2 max >10 %).33
Most studies have focused on HF with reduced ejection fraction (HFrEF). In acute HF, ID appears to be associated with both short- and intermediate-term risk of mortality.34 The presence of absolute (but not functional) ID has been shown to be independently associated with an increased risk of readmission within 30 days in a study of 693 patients admitted to hospital with acute HF.35 Interestingly, in this study over half of the patients had HF with preserved ejection fraction (HFpEF). The adverse effects of ID are less well described in HFpEF; however, in the large multicentre study by Klip et al. there was no significant interaction between ejection fraction and the impact of ID (p=0.3), although this may have been due to numbers as only 13 % of the patients included had preserved ejection fraction.8 More recently a study of 1,197 patients, including 229 with HF with mid-range ejection fraction and 72 with HFpEF, found that ID was associated with lower exercise capacity and increased mortality regardless of left ventricular ejection fraction (LVEF).36 These results suggest that iron replacement may be of benefit in HFpEF and acute HF patients as well as HFrEF patients, and have led to the growing interest in iron replacement to improve outcomes.
Iron Replacement Therapy in Heart Failure
Issues with Oral Iron Replacement
Oral iron therapy, most commonly given as ferrous sulphate or ferrous fumarate, is relatively inexpensive and widely used. However, there are limitations with oral administration, not least the low gastrointestinal absorption of iron and its limited tolerability. This intolerance is produced, at least in part, by oxidative damage of the mucosal boundary when iron is oxidised from Fe2+ to Fe3+.37 This causes side-effects such as constipation, diarrhoea and dyspepsia in up to 60 % of patients prescribed oral iron. Additionally, oral iron can take a long time to replenish iron stores, particularly if there is ongoing iron loss at the same time.
It appears that oral iron is unlikely to be efficacious in HF patients. A recently-published randomised trial by Lewis et al. evaluated 225 patients with HFrEF and ID (absolute or functional) who were given 16 weeks of oral iron or placebo and found no difference in exercise capacity (peak VO2 or 6-minute walk test), NT-proBNP or Kansas City Cardiomyopathy Questionnaire.38 In addition to this, there was minimal improvement in iron stores in the group given iron, suggesting that oral iron replacement is unlikely to be of benefit. A much smaller trial randomised 18 patients with ID anaemia to receive intravenous (IV) iron (iron sucrose 200 mg weekly for 5 weeks), oral iron (ferrous sulphate 600 mg/day for 8 weeks) or oral placebo and found that although there was no significant difference in the increase in haemoglobin between the groups, VO2 only increased in the group given IV iron.39 The results from these trials are consistent with prior trials where oral iron has been given with erythropoietin or darbepoetin, where in the groups given oral iron therapy alone there have been no significant improvements in exercise capacity or symptoms.40,41 Given these results, focus has now switched to IV iron replacement.
Intravenous Iron Replacement: Current Evidence and Guidelines
IV iron replacement avoids the gastrointestinal tract, improving absorption of iron. While oral iron therapy appears to be ineffective in HF patients with ID, there is an increasing amount of evidence suggesting that IV iron replacement is beneficial. Several small trials have suggested a potential benefit of IV iron in HF patients. Bolger et al. reported on 16 patients given IV iron sucrose for up to 17 days and found that there was an improvement in exercise capacity and some symptomatic benefit.42 The first randomised trial of IV iron, performed in 40 patients by Tobili et al., reported a reduction in NT-proBNP in HF patients with ID anaemia and renal impairment, as well as improvements in LVEF, 6-minute walk test and symptoms.43 These results were further extended to include non-anaemic patients by Okonko et al., who again found an improvement in exercise capacity and symptoms after 16 weeks of IV iron compared to placebo.44
The largest trial reported to date was the Ferinject® Assessment in patients with IRon deficiency and chronic Heart Failure (FAIR-HF) study.45 This was a randomised trial of 459 patients with HF (LVEF <45 %) and ID (absolute or functional) with haemoglobin in the 95–135 g/l range randomised to ferric carboxymaltose or placebo. The study met its primary endpoint, with those patients receiving IV iron significantly more likely to have improved self-reported Patient Global Assessment at 6 months (50 % versus 28 %, p<0.001). There were also significant improvements in NYHA class and 6-minute walk test. These results were independent of the presence of anaemia. These results were replicated in the Ferric CarboxymaltOse evaluatioN on perFormance in patients with IRon deficiency in coMbination with chronic Heart Failure (CONFIRM-HF) trial, a study of 304 patients with HF and ID in which participants were randomised to IV ferric carboxymaltose or placebo. The CONFIRM-HF trial authors reported significant improvements in 6-minute walk test, NYHA class and QoL, as well as time to first hospitalisation.46 Most recently, the Effect of Ferric Carboxymaltose on Exercise Capacity in Patients with Chronic Heart Failure and Iron Deficiency (EFFECT-HF) trial evaluated 172 patients with HF and ID and also suggested an improvement in peak VO2 with IV iron replacement.47 However, there were issues with missing data in this study.
Two meta-analyses of randomised trials of IV iron in HF patients with ID have been performed recently to summarise the results of these trials: a standard meta-analysis of five randomised trials including 509 patients and 342 controls,48 and an individual patient data meta-analysis including 504 patients and 335 controls.49 These studies have suggested a significant reduction in all-cause mortality, cardiovascular hospitalisation and HF hospitalisation with IV iron, as well as significant improvements NYHA class, 6-minute walk test and symptom questionnaire scores.
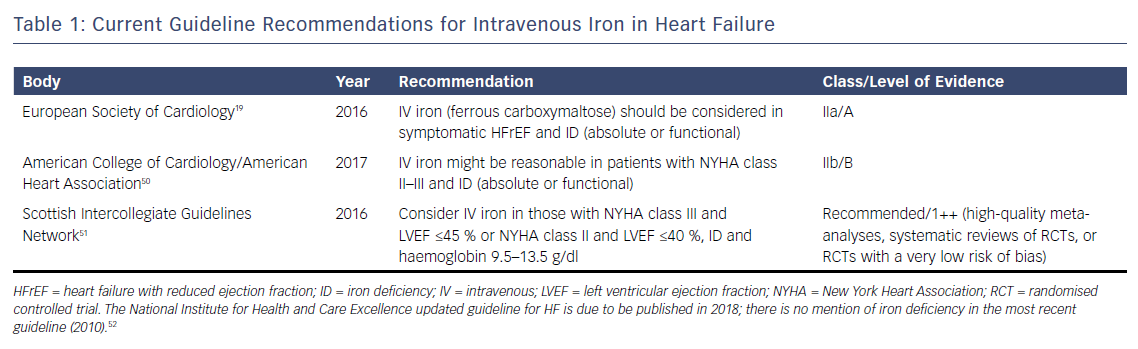
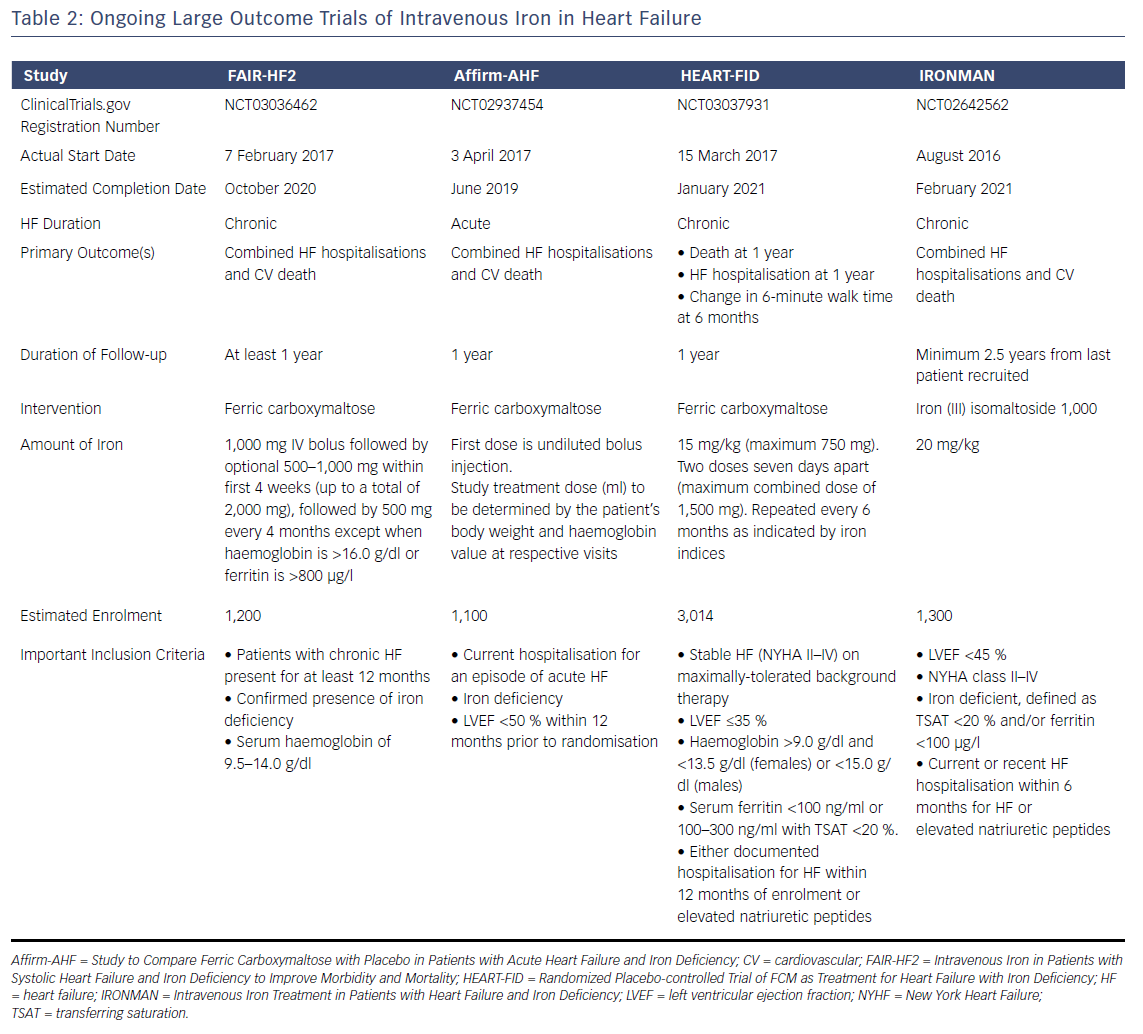
The weight of evidence from these randomised trials has led several guideline groups to recommend the consideration of IV iron therapy, (see Table 1). Nevertheless, evidence is still awaited from large outcome trials to determine the long-term prognostic benefit of IV iron replacement in HF. Several of these trials are currently ongoing, (see Table 2), and are likely to report in the near future. Positive results are likely to lead to stronger guideline recommendations to implement IV iron replacement in routine clinical practice.
Future Directions and Conclusions
As well as investigating the potential benefit of IV iron on mortality in HF, there are several other questions that remain. First, while the majority of iron replacement studies in HF have included HFrEF patients, whether IV iron replacement is of benefit in HFpEF is unclear. The ongoing FAIR-HFpEF trial will provide further insight on this (NCT03074591). Second, the efficacy and safety of IV iron replacement in the in-patient setting in acute HF is unknown. Third, whether alternative methods for the diagnosis of ID, such as ssTR, provide any benefit over and above ferritin and transferrin saturations has not been fully evaluated. Fourth, the majority of published studies performed have evaluated ferric carboxymaltose; whether other IV iron preparations provide any benefit, as well as the optimal dose and duration, are yet to be confirmed.
In conclusion, ID is common in patients with HF, and may well be underdiagnosed in routine clinical practice. Several trials of IV iron replacement have suggested benefits on exercise capacity and symptoms, so IV iron should be considered in symptomatic HF patients with ID. Several ongoing trials will provide further evidence as to the long-term effects of such treatment on mortality and hospitalisation.