The renin–angiotensin–aldosterone system (RAAS) is an essential regulatory component of cardiovascular homeostasis that exerts its actions through hormones, angiotensin II and aldosterone, which regulate vascular tone and blood pressure by causing vasoconstriction and renal sodium and water retention.1 Abnormalities in cardiac function in heart failure (HF) activate the RAAS and sympathetic nervous system in order to maintain perfusion of vital organs.2 Prolonged activation of these systems causes myocardial hypertrophy, fibrosis and apoptosis, increased systemic vascular resistance, increased sodium and water retention, and potassium excretion. This has led to the therapeutic use of RAAS inhibitors in HF,3,4 including angiotensin-converting enzyme inhibitors (ACEIs),5 angiotensin receptor blockers (ARBs)6 and mineralocorticoid receptor antagonists (MRAs).7 These drugs have become the mainstay of current therapy in heart failure with reduced ejection fraction (HFrEF).
While RAAS inhibitors have substantial clinical benefit in patients with HFrEF, they interfere with the stimulatory effect of angiotensin II on aldosterone secretion in the adrenal gland,8 resulting in decreased aldosterone concentrations, decreased delivery of sodium to the distal nephron, abnormal collecting tubule function and impaired renal excretion of potassium, leading to hyperkalaemia.9 This is particularly common in patients with hypovolemia and worsening of renal function. Furthermore, physiological changes associated with HF progression may influence the pharmacokinetics and pharmacodynamics of all drugs used in patients with HF.10 As a result of the risk of hyperkalaemia, many patients are maintained on suboptimal RAAS inhibitor therapy (both in terms of individual drug classes and daily dose) or even discontinuing therapy. Clinical data suggest that higher doses of RAAS inhibitors than those currently in use might be most effective11,12 but at present this cannot be tested because of the risk of inducing hyperkalaemia.
This article aims to discuss the management of HF patients who require RAAS blockade but are at risk of worsening renal function and hyperkalaemia, as well as describing two new potassium binders that have the potential to allow more HF patients to receive optimal doses of guideline-recommended therapies.
Hyperkalaemia: Prevalence and Risk Factors
Hyperkalaemia is defined as serum potassium concentration >5.0 mEq/l and may result from extracellular shifts of potassium, excessive ingestion of potassium and/or impaired elimination of potassium by the kidneys.13 The European Society of Cardiology guidelines advise caution in the initiation of ACEIs or ARBs if potassium levels exceed 5.0 mEq/l and state that if during therapy potassium rises to >5.5 mEq/l, the ACEI or ARB should be adjusted or stopped and specialist advice sought.14 Laboratory monitoring of potassium levels after MRA initiation frequently does not meet guideline recommendations, even in patients at higher risk for complications or in a specialised setting, placing patients at risk of hyperkalaemia that goes unnoticed.15
In clinical trials evaluating RAAS inhibitor therapy in patients with HFrEF, such as the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF) and Randomized Aldactone Evaluation Study (RALES), severe hyperkalaemia (defined as serum potassium concentration ≥6.0 mEq/l) has been reported in around 2.0–2.5 % of participants.7,16 Hyperkalaemia of any degree has been reported in 10 % of patients within a year of initiating RAAS blockade and is severe in approximately 1 % of patients with diabetes.17–19 However, patients with baseline renal dysfunction or hyperkalaemia were excluded from these clinical trials and there are strict protocols in place to minimise risk of significant side effects in such trials. In more advanced stages of chronic HF, as renal function begins to decline the risk of hyperkalaemia increases significantly; an incidence of between 5 and 10 % has been reported in patients with chronic kidney disease (CKD), and this increases with disease stage.20,21 Hypokalaemia is also highly prevalent in older HF patients. In the Trial of Intensified versus Standard Medical Therapy in Elderly Patients with Congestive Heart Failure (TIME-CHF), in which patients ≥60 years of age (n=586) were randomised to a standard versus an intensified N-terminal brain natriuretic peptide-guided HF therapy, during 18-month follow up hyperkalaemia (≥5.5 mEq/l) was reported in 13.4 % of patients, with severe hyperkalaemia (≥6.0 mEq/l) in 4.9 %.22
Renal dysfunction is a common comorbidity in HF patients.23 A study of 552 hospitalised HF patients reported a mean estimated glomerular filtration rate (eGFR) of 66.9±30.4 ml/min/m², severe kidney failure (eGFR<30 ml/min/1.73 m²) in 8.0 %, moderate kidney failure (eGFR 30–60 ml/min/1.73 m²) in 35.5 %, and an eGFR of >60 ml/min/1.73 m² (no/mild kidney failure) in 56.5 %.24 In the European Society of Cardiology HF Pilot study, a prospective, multicentre, observational survey conducted in 136 cardiology centres in 12 European countries (n=5,118), severe kidney failure was reported in 9.9 % of patients with acute HF and 5.1 % with chronic HF; eGFR of >60 ml/min/1.73 m² was reported in 49.3 % and 40.6 % of acute and chronic HF patients, respectively.25 In the EuroHeart Failure Survey of 3,658 patients with HF due to left ventricular systolic dysfunction, eGFR <60 ml/min/1.73 m² was reported in 52.6 % of participants. Renal dysfunction was associated with lower prescription of ACEIs at discharge (74 % versus 83 %, p<0.001).26
The difference between controlled clinical studies and routine clinical practice was illustrated by RALES, which investigated the MRA spironolactone in patients with HF and serum creatinine <2.5 mg/dl. This study reported only a 2 % incidence of hyperkalaemia,7 but following its publication abrupt increases were seen in the rate of prescriptions for spironolactone and in hyperkalaemia-associated morbidity and mortality.27 There is also a dose-dependent increase in the risk of hyperkalaemia with escalation in RAAS inhibitor therapy. In the RALES dose-finding study, the incidence of hyperkalaemia was 5 % with 12.5 mg spironolactone but was 24 % with a 75 mg dose.28
The EMPHASIS-HF study investigated the safety and efficacy of the MRA eplerenone in patients with systolic HF and mild symptoms (New York Heart Association functional class II). Eplerenone reduced cardiovascular death or HF hospitalisation when added to evidencebased therapy (including RAAS inhibitors and beta-blockers), although hyperkalaemia was reported as an adverse effect (8 % in the eplerenone group versus 3.7 % in the placebo group; p<0.001).16 In an analysis of high-risk subgroups, patients treated with eplerenone had an increased risk of potassium >5.5 mEq/l, but not of potassium >6.0 mEq/l, and of hospitalisation for hyperkalaemia.29 Despite this, the incidence of hyperkalaemia did not eliminate the survival benefit of eplerenone.30 Serum potassium levels and renal function should be assessed prior to initiating eplerenone therapy and periodic monitoring should be undertaken, especially in patients at high risk of developing hyperkalaemia.31
In a recent secondary analysis of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure (PARADIGM-HF) study, severe hyperkalaemia (potassium >6.0 mEq/l) was more common in patients randomly assigned to enalapril than to sacubitril/valsartan (3.1 versus 2.2 per 100 patientyears; hazard ratio: 1.37, 95 % CI [1.06–1.76]; p=0.02).32 These data suggest that neprilysin inhibition attenuates the risk of hyperkalaemia when MRAs are combined with other RAAS inhibitors in patients with HF.
The exclusion from clinical trials of patients who are at the greatest risk for hyperkalaemia means that its true incidence may have been underestimated: in a study at an outpatient centre of 1,818 patients using ACEIs, 11 % of patients developed hyperkalaemia.33 In addition to this, patients in clinical trials are closely monitored, including frequent blood tests and clinical visits. As a result, rising serum potassium levels are more likely to be addressed at an early stage.
Risk factors for hyperkalaemia in patients with HF include: age >65 years; comorbidities that impair kidney function and decrease eGFR, including diabetes (in which a prevalence of 15 % has been reported)34 and kidney disease (e.g. acute or chronic tubulointerstitial renal disease, diabetic nephropathy, renal transplants, urinary tract obstruction, systemic lupus erythematosus and sickle cell disease); current use of potassium-sparing diuretics, excessive potassium ingestion and the use of several concomitant medications including non-steroidal anti-inflammatory drugs (NSAIDs), antimicrobial drugs and beta-blockers.17,35–37 NSAIDs may also impair renal function in patients with HF.38 Since diabetes, CKD and HF occur together in many patients, hyperkalaemia presents a significant clinical challenge and an unfortunate paradox: while RAAS inhibitor therapy reduces morbidity and mortality in patients with HF, it increases the risk of hyperkalaemia in these already high-risk patients.
The clinical consequences of hyperkalaemia are severe: arrhythmias and asystole that may lead to cardiac arrest and sudden death.39,40 Hyperkalaemia is responsible for increased morbidity, mortality and hospitalisations in patients with HF, particularly in older patients with comorbidities.27,36,41 Severe hyperkalaemia has a mortality rate of up to 30 % if not treated rapidly.42 Interestingly, one study found that the association between hyperkalaemia and mortality was no longer significant if the plasma potassium decreased by ≥1 mEq/l within 48 h of admission to the critical care unit.43 In addition to the direct risk imposed by increased serum potassium, intermittent hyperkalaemia has a more serious negative impact on clinical outcomes because its treatment usually involves discontinuation or dose reductions of lifesaving drugs.44 Although clinical data show that up-titrating RAAS inhibitors confers significant clinical benefit,11,12 the dosage of RAAS inhibitors is often suboptimal in everyday clinical practice,45,46 with one study reporting that less than one-third of eligible patients hospitalised for HF received guideline-recommended aldosterone antagonist therapy.47 Underutilisation of RAAS inhibitors results largely from clinician fears of adverse effects including hyperkalaemia.48 There is therefore a need for effective treatment options for hyperkalaemia.
Treatment Options for Hyperkalaemia
Therapeutic goals for patients with acute hyperkalaemia are to reverse adverse cardiac effects, shift potassium into the cells, eliminate potassium from the body and normalise serum potassium levels. Acute treatments include 25–50 % intravenous glucose, calcium gluconate or chloride, insulin, sodium bicarbonate, beta-2-agonists, diuretics, cationexchange salts and haemodialysis.49 However, these treatments are temporary and often inadequate; costly and invasive haemodialysis is usually needed for patients with severe hyperkalaemia.50
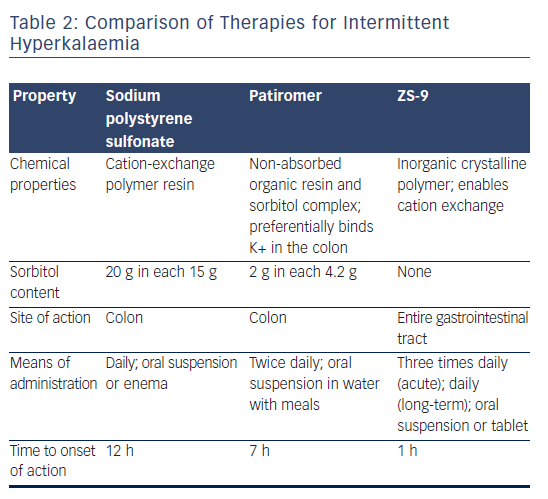
For >50 years, sodium polystyrene sulfonate (SPS), a cation-exchange resin that binds potassium in the colon, has been used in the longterm lowering of serum potassium levels.51 It is, however, intolerable to many, has never been tested in a randomised controlled trial and there are questions regarding its efficacy and safety.52 The administration of SPS alone can lead to severe constipation and impaction, which have led to its co-administration with sorbitol. Treatment with SPS and sorbitol, however, has been shown to cause potentially fatal colonic necrosis.52,53 SDS has a boxed warning for colonic necrosis and its use is contraindicated in patients who do not have normal bowel function.54 Despite concerns related to colonic necrosis, this serious complication appears to be rare: a retrospective cohort study found an incidence of 0.14 % in 2,194 patients prescribed SPS versus 0.07 % in those not prescribed SPS (p=0.2).
Other strategies for the control of intermittent hyperkalaemia include the restriction of dietary potassium, but this leads to poor dietary adherence and limits healthy food choices that are associated with beneficial cardiovascular outcomes.55 Patients with HF are advised to restrict sodium intake, which leads to them using salt substitutes that are rich in potassium – a case was reported in The Lancet in 2013.56 For many years there remained an unmet need for a hyperkalaemia therapy that was effective, safe and well tolerated.
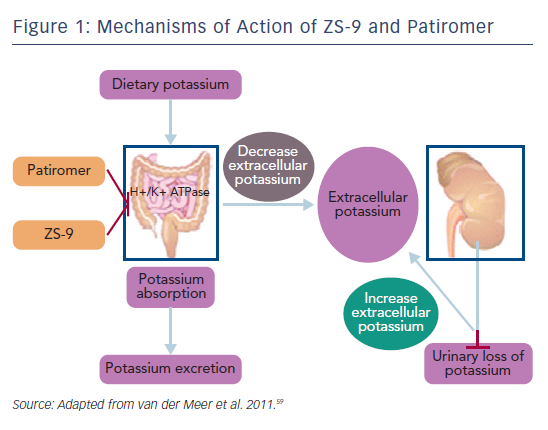
In October 2015 the US Food and Drug administration approved the first new potassium binder for the treatment of hyperkalaemia in >50 years.57 Patiromer is an organic, high-capacity cation-exchange polymer in the form of its calcium salt complexed with sorbitol (ratio 2:1), and exchanges calcium for potassium as it moves through the colon, preventing the reabsorption of potassium and facilitating its elimination in the faeces (see Figure 1).58,59 It is a dry, odourless, tasteless powder that has a low viscosity, consisting of small (100 µm) uniform beads that swell minimally when suspended in water, and is typically administered in 40 ml of water with a meal.58 It does not require co-administration with a laxative and is more palatable than SPS.
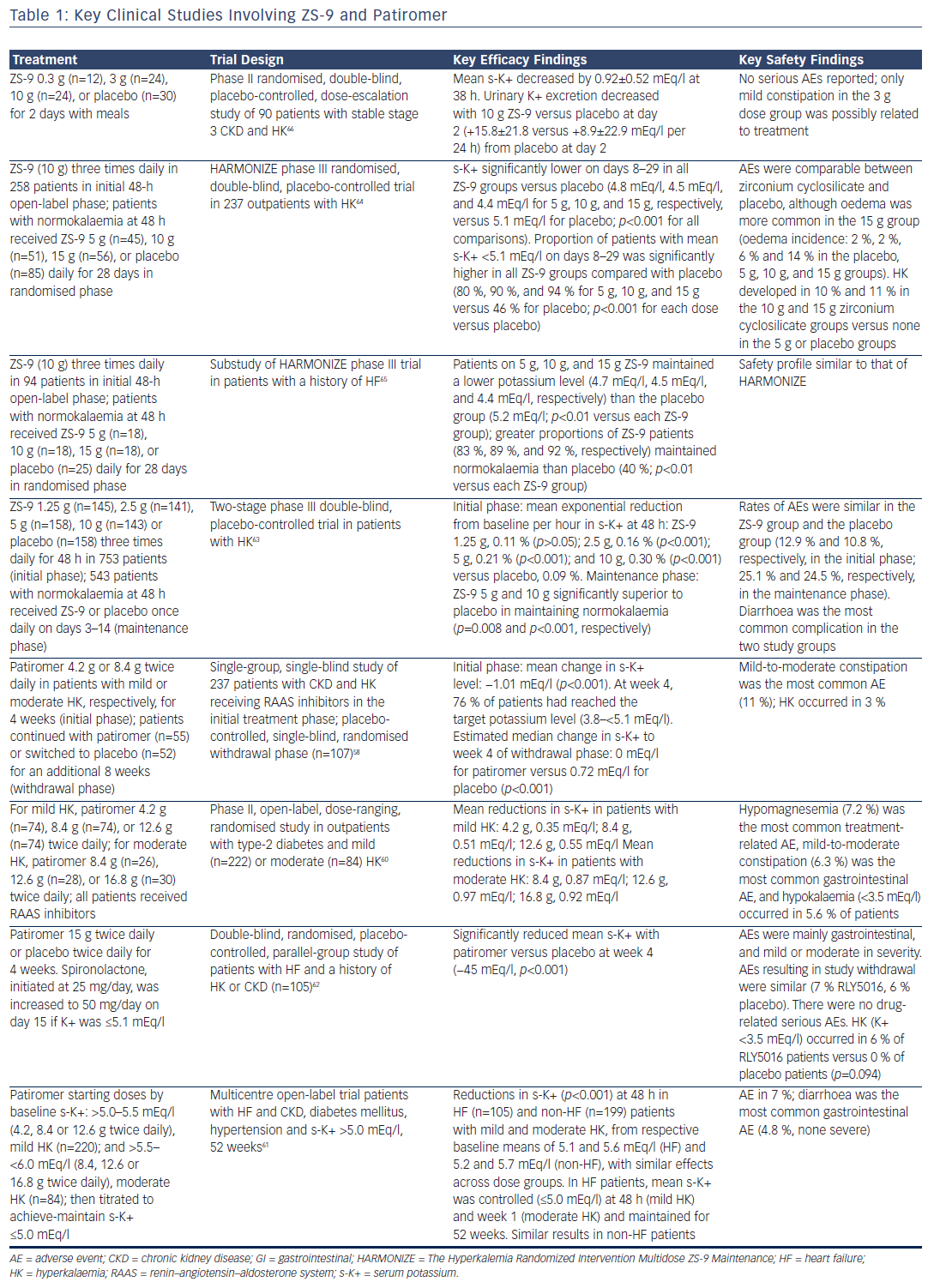
Four clinical studies have demonstrated the efficacy of patiromer in decreasing serum potassium, preventing the recurrence of hyperkalaemia and reducing RAAS inhibitor discontinuation (see Table 1). RLY5016 in the Treatment of Hyperkalemia in Patients With Hypertension and Diabetic Nephropathy (AMETHYST-DN), was a phase II, multicentre, randomised, open-label, dose-ranging study of patients (n=304) with type 2 diabetes and CKD stage 3 or above (eGFR 15 ml to <60 ml/min/1.73 m2) and serum potassium >5.0 mEq/l in the setting of RAAS inhibitor dose optimisation for blood pressure control, or who were on a RAAS inhibitor and had serum potassium >5.0 mEq/l at the time of screening. After 4 weeks, patients in the patiromer group showed statistically significant decreases in serum potassium levels, which were maintained throughout the 52-week treatment period.60
The Two-Part, Single-Blind, Phase III Study Evaluating the Efficacy and Safety of Patiromer for the Treatment of Hyperkalemia (OPAL-HK) trial was a phase III study that evaluated the efficacy and safety of patiromer for the treatment of hyperkalaemia in patients with stage 3 or 4 CKD on ≥1 RAAS inhibitor. By week 4 of the initial treatment phase, three-quarters of patients taking patiromer achieved normal potassium levels.58 Finally, a multicentre, open-label 52-week trial evaluated patiromer in patients with HF and CKD, type 2 diabetes and hypertension. Patiromer reduced and maintained mean serum potassium ≤5.0 mEq/l for up to 1 year in HF and was well tolerated.61
Patiromer has also been evaluated for the prevention of hyperkalaemia in patients. The Evaluation of RLY5016 in Heart Failure Patients (PEARLHF) was a multicentre, randomised, double-blind, placebo-controlled parallel group multiple-dose study in patients receiving standard therapy and spironolactone (n=105). Spironolactone was given to both groups at 25 mg/day and increased to 50 mg/day if serum potassium was ≤5.1 mEq/l. From day 3, the patiromer group had significantly lower serum potassium levels than the placebo group, and at the end of the treatment period (week 4) the patiromer group had a significantly lower incidence of hyperkalaemia. In addition, the administration of patiromer enabled the dosage of spironolactone to be up-titrated while still maintaining normokalaemia.62
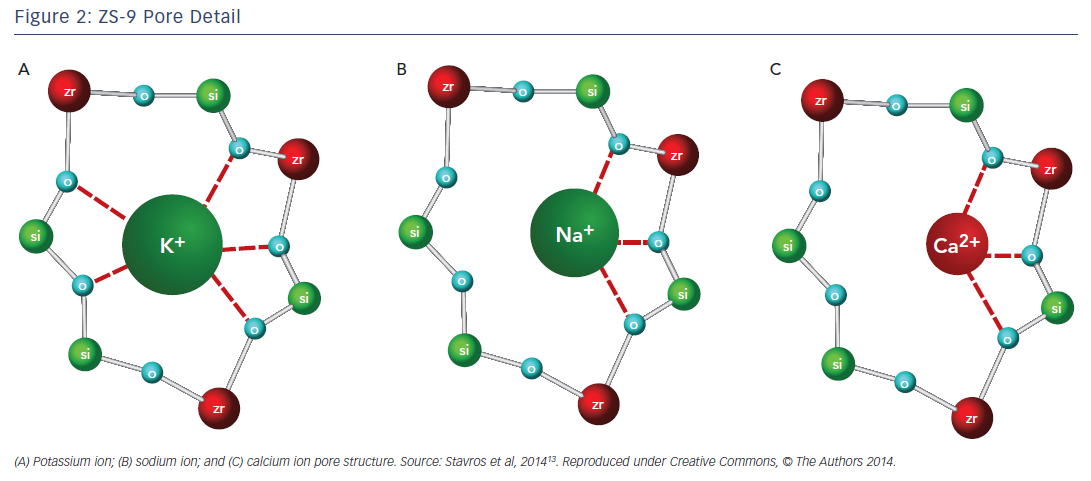
Another agent in clinical development, sodium zirconium cyclosilicate (ZS-9), is an orally-administered, insoluble, non-absorbed inorganic sodium–potassium cation exchange agent that is >125 times more selective for potassium ions than SDS in vitro.13 The potassium-lowering action of ZS-9 is based on size-selective micropores in the zirconium silicate crystalline lattice structure that trap potassium in the intestinal tract (Figure 2).13 It has been hypothesised that the hydronium sites are responsible for the majority of potassium binding, exchanging protons for potassium ions, leaving most of the sodium ions bound to ZS-9 in a less exchangeable site. The exact mechanism of how the exchange occurs, however, has not been determined.13 Preclinical studies suggest that ZS-9 acts throughout the entire gastrointestinal tract.13
ZS-9 has demonstrated excellent efficacy in three large clinical trials (see Table 1). In a two-phase dose-finding phase III study, patients with hyperkalaemia (n=753) were randomised to receive either ZS-9 or placebo three times daily for 48 h. Patients who achieved normokalaemia (serum potassium 3.5–4.9 mEq/l) at 48 h were randomly assigned to receive either ZS-9 or placebo once daily on days 3–14 (maintenance phase).63 The Hyperkalemia Randomized Intervention Multidose ZS-9 Maintenance (HARMONIZE) study was a randomised, double-blind, placebo-controlled phase III trial with an open-label phase (n=258) and a randomised phase (n=257). The use of ZS-9 normalised serum potassium within a median of 2.2 h and normal potassium levels were maintained for up to 28 days in a high proportion of patients on all ZS-9 doses. Patients with higher baseline serum potassium levels experienced greater reductions. All doses of ZS-9 reduced serum aldosterone, and the efficacy was not affected by the use of RAAS inhibitors.64 In a substudy of HARMONIZE, HF patients with evidence of hyperkalaemia (serum potassium ≥5.1 mEq/l, n=94) were treated with open-label ZS-9 for 48 h. Patients (n=87; 60 receiving RAAS inhibitors) who achieved normokalaemia were randomly assigned to daily ZS-9 or placebo for 28 days. The majority of patients (69 %) in this study received RAAS inhibitors while taking ZS-9 and maintained normal serum potassium levels without adjustment of their RAAS inhibitor dose.65 In addition, a phase II randomised, double-blind, placebo-controlled, dose-escalation study evaluated ZS-9 in HF patients with stable stage 3 CKD and hyperkalaemia (n=90). ZS-9 caused a rapid, sustained reduction in serum potassium and was well tolerated.66
The long-term safety and efficacy of ZS-9 are being evaluated in an ongoing open-label phase III study, ZS-005. Patients (n=751) with hyperkalaemia (defined as serum potassium ≥5.1 mEq/l) received an induction regimen of ZS-9 10 g three times daily for 24–72 h. Those whose potassium reached normal levels on the study drug – a serum potassium level of 3.5–5.0 mEq/l – received a maintenance regimen of ZS-9 5 g/day for a maximum of 12 months. Interim results for 436 patients who had completed at least 6 months of treatment were presented recently. The mean serum potassium was 4.7 mEq/l, and almost 90 % of patients had a mean serum potassium ≤5.1 mEq/l over months 3–12.67
The US Food and Drug Administration issued a Complete Response Letter regarding the New Drug Application for ZS-9 in May 2016 due to observations arising from a pre-approval manufacturing inspection;68 however, resubmission is expected.
Comparison of Potassium-binding Agents
A comparison of the available potassium-binding agents is given in Table 2. Of the three, ZS-9 and patiromer offer greater potassiumbinding selectivity than SDS and are unaffected by the presence of calcium or magnesium ions.13,58 ZS-9 has the fastest onset of action: within 1 h64 compared to within 12 h for SPS69 and within 7 h for patiromer.70 A combined analysis of both phase III trials of ZS-9 focused on the short-term changes in serum potassium of receiving an initial 10 g dose of ZS-9 in a subpopulation of patients with severe hyperkalaemia (defined as serum potassium 6.1–7.2 mEq/l). The mean serum potassium was reduced by 0.4 mEq/l at 1 h, 0.6 mEq/l at 2 h and 0.7 mEq/l at 4 h. The median time to serum potassium levels <6 and 5.5 mEq/l were 1.07 and 4 h, respectively. No cases of hypokalaemia or adverse effects were reported during the first 48 h of ZS-9 therapy.71 ZS-9 may therefore be useful in the acute setting.
All three agents are available as oral suspensions, although SPS can also be used as an enema.72 SPS may be administered one to four times daily, depending on the desired total dose. In clinical trials, patiromer was administered twice daily for maintenance therapy,58,60–62 whereas ZS-9 was administered once daily.63,64 However, in acute use for initial lowering of potassium over 48 h, ZS-9 was typically given three times daily.64,66
SDS when administered alone is associated with severe gastrointestinal side effects, making it unsuitable for routine use. Adverse effects with patiromer include mild-to-moderate constipation (up to 11 %), hypokalaemia (5–6 %) and hypomagnesemia (3–24 %), but these have not been reported to be serious. For ZS-9, mild constipation/ diarrhoea (2–8.7 %), hypokalaemia (around 10 % depending on dose) and oedema (2–4 %) have been reported. The latter was not serious in the clinical studies but, given the relationship between oedema and HF, patients should be monitored.
Practical Aspects of Treatment with Potassium-binding Agents
It is essential that the use of these therapies does not result in less stringent monitoring of patients’ potassium levels. Patients with HF and renal failure and at risk for hyperkalaemia need very close clinical follow-up, including laboratory testing, even if they are taking potassium binders. In addition, ZS-9 and patiromer require close monitoring for hypokalaemia, and ZS-9 requires monitoring for oedema. It is important to educate patients in the importance of adherence to these therapies; the once-daily dosing of ZS-9 may be advantageous in this respect. Although clinical studies to date have not identified significant drug– drug interactions, one potential complication of these agents is their ability to bind other drugs. Patiromer binds furosemide, metoprolol and amlodipine, which are among the most common drugs prescribed to CKD patients. Patiromer carries a boxed warning stating that there should be a 6-h window between taking any orally-administered medication and patiromer.57
Summary and Concluding Remarks
The management of hyperkalaemia in patients taking RAAS inhibitors in HF is challenging, and often leads to suboptimal use of these lifesaving drugs, resulting in increased morbidity and mortality. Clinical trial data indicate that patiromer and ZS-9 are effective, safe and predictable options for the treatment of hyperkalaemia, as well as the maintenance of normokalaemia, without the dose reduction or discontinuation of RAAS inhibitors. In addition, ZS-9 offers the potential for acute therapy in conscious patients instead of insulin and glucose. Acute dialysis for the sole indication of hyperkalaemia may also be eliminated.
It must be stressed that patiromer has been shown to demonstrate safety and efficacy over 52 weeks, but long-term safety data for ZS-9 are still not available; studies are currently ongoing. Further long-term, randomised-controlled trials will be needed to determine whether patiromer and ZS-9 can increase the use of optimum doses of RAAS inhibitors and improve outcomes in patients with HF. Future studies should include specific patient cohorts, including patients with severe hyperkalaemia, more advanced HF, those with a preserved ejection fraction and with severe CKD. Since hyperkalaemia is often diagnosed in the hospital setting, hospitalised patients should also be observed in future trials. Hyperkalaemia can develop during transient worsening of renal function, a relatively common occurrence during RAAS initiation/up-titration, and this warrants further investigation. There is a need for studies to determine whether there are any issues such as fluid and electrolyte or acid–base changes, drug–drug interactions or longterm tolerability that might influence the choice of patiromer or ZS-9 in specific patient populations or indications. There is also a need for more information on the long-term efficacy and safety of both drugs in real-world settings in patients with other comorbidities and taking multiple drugs.
To date, ZS-9 and patiromer have only been evaluated in the treatment of hyperkalaemia, but they may find a more important role in the prevention of hyperkalaemia. At present, many patients with moderate CKD who have high serum potassium levels, around 4.8 or 4.9 mEq/l, are not given RAAS inhibitors because of the fear of causing hyperkalaemia. These agents may be beneficial in patients who have previously taken RAAS inhibitors but discontinued them after experiencing a single hyperkaliaemic event. They may also enable the use of RAAS inhibitors in patients with risk factors for hyperkalaemia. There is a need for further study investigating the optimisation of RAAS inhibitors in such patients. The fast onset of ZS-9 suggests that it may be an effective treatment option for acute hyperkalaemia. Again, further investigation is required. However, caution should be exercised in prescribing potassium binders; they should be given only to selected patients.
In conclusion, the use of patiromer and ZS-9 represents an important development in HF therapy, potentially enabling patients with HF to optimise the use and dose of RAAS inhibitors, improve dietary choices, improve quality of life and possibly reduce morbidity and mortality in these high-risk populations.