According to the third report from the International Society for Heart and Lung Transplantation (ISHLT) Mechanically Assisted Circulatory Support Registry, more than 15,500 left ventricular assist devices (LVADs) were implanted worldwide between 2013 and 2017.1 Originally, LVADs were exclusively implanted as part of a bridge to transplantation (BTT) strategy, but in recent years a large proportion of patients undergoing LVAD implantation received the device as final treatment for advanced heart failure (HF), so-called destination therapy.
Survival rates after LVAD implantation have increased greatly over the past decade, as improvements in LVAD technology and management have resulted in a lower risk of adverse events.1–7 Hence, in recent studies, 2-year survival rates range from 80% to 90%.5,8 Patients treated with LVADs experience significant improvements in HF symptoms, quality of life (QoL) and functional capacity (FC), although the latter remains reduced in this patient group, especially when measured as peak oxygen uptake (pVO2).3,6–11
Given the increased use of LVAD as destination therapy and the long wait times for transplantation in those implanted with an LVAD as BTT, optimisation of FC and QoL is critically important.1 In order to identify potential strategies to improve FC and QoL after LVAD implantation, a detailed understanding of the mechanisms behind residual impairment of these parameters is essential.
This review summarises the available evidence describing improvement in FC and QoL after LVAD implantation with the specific aim of identifying reproducible predictors of improvement in QoL and FC with the intervention.
Methods
Search Strategy
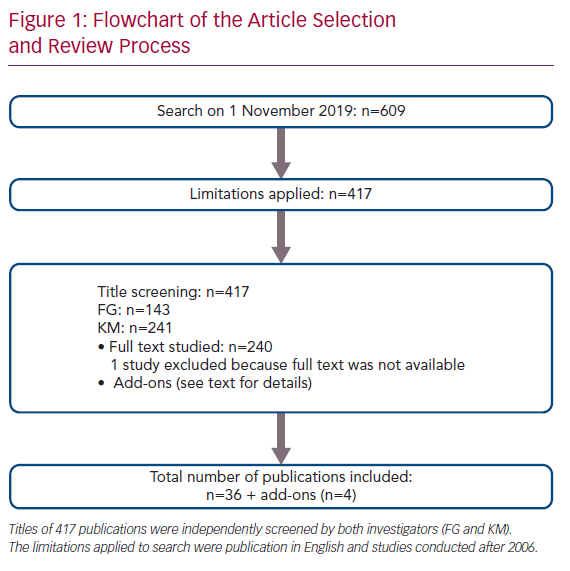
On 1 November 2019, a PubMed search was conducted using the search terms ‘quality of life’, ‘EuroQoL-5 Dimensions-5 levels’, ‘Minnesota Living With Heart Failure’, ‘Kansas City Cardiomyopathy Questionnaire’, ‘exercise’, ‘six minute walk test’, ‘six-minute walk test’, ‘peak oxygen uptake’, ‘exercise capacity’, ‘peak oxygen consumption’, ‘exercise training’, ‘cardiac rehabilitation’, ‘ventricular assist device’ and ‘continuous-flow left ventricular assist device’ (Supplementary Material Table 1). The search resulted in 609 items (Figure 1). After excluding studies published prior to 2006 and those written in languages other than English, the titles of 417 publications were screened by both investigators independent of each other (i.e. blinded). This process resulted in the identification of 143 publications by FG and 241 publications by KM. Mismatch and disagreement in 98 cases led to full-text review. The full text of one article could not be acquired, which resulted in its exclusion. Finally, 36 papers and four ‘add-on’ studies (e.g. extra studies found by reference review or other sources) were included in this narrative review.9,12–50
Calculations
Mean values weighted for population size for pVO2 and the 6-minute walk test (6MWT) were calculated as follows:
Weighted mean pVO2 = Σ(pVO2 × npVO2)/ΣNpVO2
Weighted mean 6MWT = Σ(6MWD × n6MWT)/ΣN6MWT
Where n refers to the number of patients in each study, while N is the total number of all studied patients. 6MWD is the distance covered in the 6MWT.
Results and Discussion
Functional Capacity After LVAD Implantation
The most widely accepted measures of functional capacity in HF are symptoms, measured by New York Heart Association (NYHA) class, 6MWD and pVO2. Each of these measures has its own advantages and disadvantages, such as variable reproducibility or technical demands, but all are useful and required to characterise different aspects of the exercise limitation of HF populations. Patients referred for LVAD implantation are almost invariably severely symptomatic (e.g. NYHA IIIb–IV with 6MWD <300–400 m and low pVO2). The latter is typically below 12 ml/kg/min, which is also the limit used as part of the indication for destination therapy LVAD in the US Medicare system.
In all studies of patients undergoing LVAD implantation, NYHA class improves in most patients from Class III/IV before implantation (100%) to Class I or II.9,49 Studies are remarkably consistent in finding that approximately 80% of patients are in NYHA Class I–II after implantation, and the improvement in symptoms has been documented to be sustained over time.9,20,49 However, some patients (<5%) remain severely symptomatic (NYHA Class IV) even 12 months after LVAD implantation.9,49
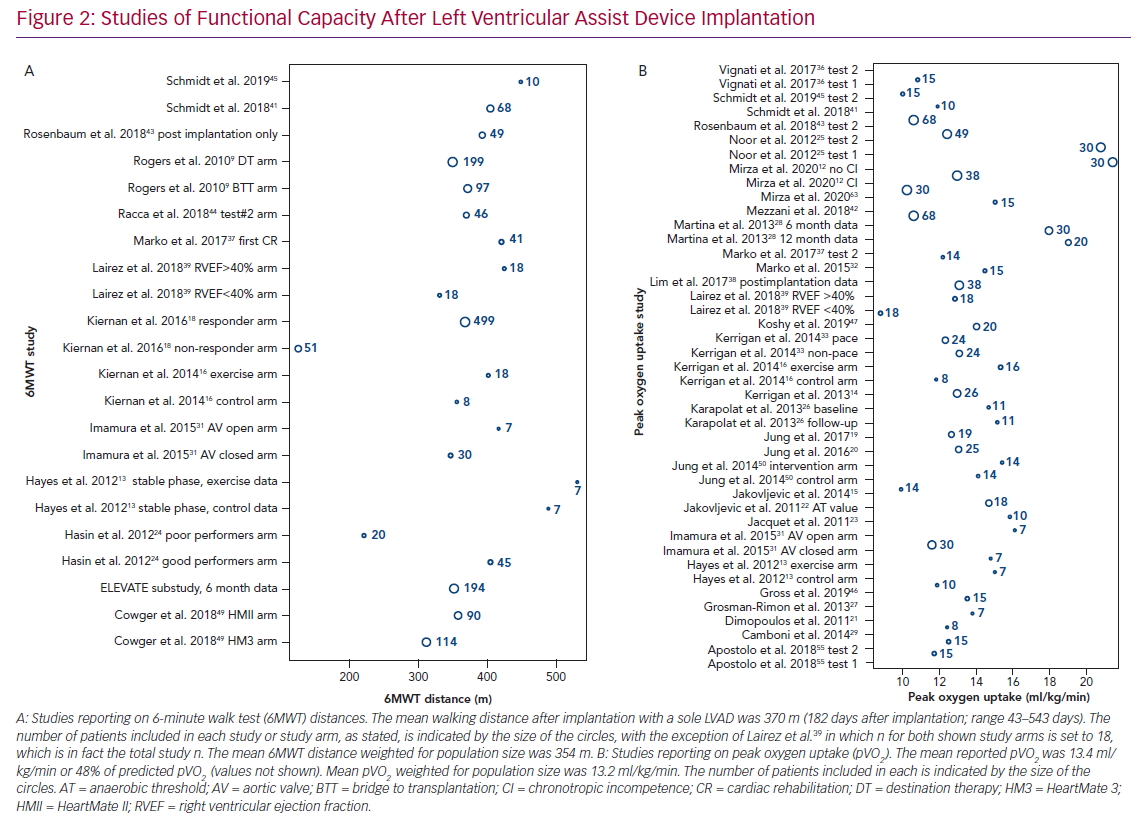
The 6MWD is widely used in LVAD recipients.9,13,16,24,31,48,49 FC measured using the 6MWT prior to implantation is low (mean 6WMD 221 m [range 39–356 m]; mean 6MWD weighted for population size 215 m), and significant improvements are observed soon after implantation (mean 6WMD 373 m [range 126–531 m]; mean 6MWD weighted for population size 357 m; Figure 2A).9,18,24,43,49 The improvement from baseline to the 6-month follow-up is, on average, +144 m (range 41–319 m; mean improvement weighted for population size 113 m), which is equivalent to an approximate 40% improvement.9,18,24,43,48,49
Improvement in the 6MWD can be difficult to interpret across different studies because some studies include mostly ambulatory patients and some include patients who would not be able to complete any exercise testing prior to implantation due to critical clinical condition (e.g. Interagency Registry of Mechanically Assisted Circulatory Support [INTERMACS] profile 1–2). In the large LVAD trials, increments in 6MWD were in the range 98–250 m from baseline to a maximum 2 years after implantation5,9,51–60 When investigating FC expressed as pVO2, the reported preimplantation values are low (mean 11 ml/kg/min [range 10.1–11.8 ml/kg/min]; mean pVO2 weighted for population size 11 ml/kg/min), although improvement is observed because studies reporting pre- and postimplantation pVO2 values show an improvement of approximately 20% after implantation.27,38,43
Postimplantation mean pVO2 values (Figure 2B) vary from 8.8 to 21.4 ml/kg/min (mean pVO2 weighted for population size 13.2 ml/kg/min), showing that pVO2 generally remains reduced after implantation at, on average, 48% of the expected value for age and sex.12–16,21–23,25–29,31–37,39–42,44–47,61–63 A considerable proportion of the variance of postimplantation pVO2 between published studies can be attributed to differences in the mean age of included patients.11
Although the improvement from pre- to postimplantation FC, measured as both 6MWT and pVO2, may appear modest, these changes are much larger than the effect of other device therapies in HF, such as cardiac resynchronisation therapy or the use of vasodilators.64,65 Furthermore, it should be re-emphasised that the sickest patients were excluded from studies presenting changes in FC from before to after implantation because these patients were not able to complete preimplantation measurements (e.g. because of the need for ventilator treatment or temporary mechanical circulatory support). Hence, the improvement in 6MWD and pVO2 from before to after implantation is often underestimated in the literature.
Preimplantation Predictors of Postimplantation Functional Capacity
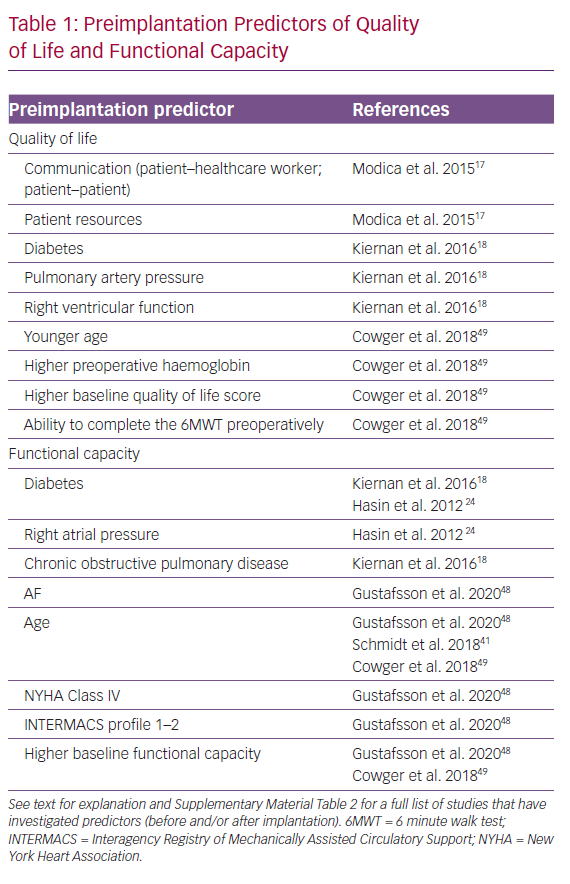
Several studies have elucidated preimplantation determinants of postimplantation QoL and/or FC (Table 1 and Supplementary Material Table 2).17,18,24,27,38,43,48,49
Advanced age is generally a predictor of inferior outcomes in cardiovascular medicine; this also holds true for patients implanted with an LVAD. In a Multicenter Study of MAGLEV Technology in Patients Undergoing Mechanical Circulatory Support Therapy with HeartMate 3 (MOMENTUM 3) substudy including 265 patients from the US, younger age was one of the strongest predictors of the ‘living well on a left ventricular assist system’ endpoint (6MWD >300 m and Kansas City Cardiomyopathy [KCCQ] score >50).49 Confirming these findings, Martina et al. showed that HeartMate II (HMII) recipients <50 years of age performed significantly better than LVAD recipients >50 years of age.28 Several other studies have confirmed age as an independent preoperative determinant of postimplantation FC.41,46,48
Other strong preoperative predictors of FC are a lower INTERMACS profile, NYHA class, chronic obstructive pulmonary disease (COPD), diabetes and lower estimated glomerular filtration rate (eGFR).15,24,24 Higher haemoglobin, eGFR and INTERMACS profile and better NYHA class at the time of implantation all reflect a general better health status, which is linked to better FC after implantation. In general, these findings are suggestive of benefits, at least in terms of FC improvement, of early implantation of LVADs in advanced HF.
The importance of perioperative diabetes has been suggested in four studies, although the largest study did not find perioperative diabetes of importance for postimplantation FC.18,24,48,66 This may be due to differences in the definitions of FC used, as well as follow-up time, and more studies dissecting the interplay between diabetes and outcome after LVAD implantation are clearly needed. These studies provide important information that can be used in the clinical setting when aligning expectations with potential LVAD recipients and their families and carers.
In general, most of the studies mentioned above investigated HMI recipients in a historical period where knowledge regarding patient selection was sparse. Further, studies including data on preoperative cardiopulmonary exercise testing (which is the gold standard for studying FC), are limited.27,38,43 A few studies evaluated preoperative predictors of postimplantation FC, with FC measured as walking ability (6MWT), and these have been discussed above.28,41,46,48,49
Relationship Between Postimplantation Functional Capacity and Adverse Events
Overall, few studies report adverse events (AEs) in relation to exercise testing in this patient group, with one case of syncope and one event of ventricular tachycardia reported, both of which were well tolerated.16,32 The potential concern of AEs occurring in relation to exercise seems clinically unimportant because exercise and cardiac rehabilitation programs have been well-tolerated in both the short and long (weeks) term.19,25,42,45,49,54,56,60
An investigation of determinants of FC in 204 patients at 6 months after implantation in a substudy of the MOMENTUM 3 trial (HeartMate 3 [HM3]=114, HMII=90) found that individuals with no severe AEs (SAEs) had larger improvements in walking distance than those who experienced an SAE (e.g. the presence of a single SAE was associated with less improvement in walking distance regardless of device type).49 Similarly, Imamura et al. showed that an increased pVO2 was associated with lower readmission rates, underlining the clinical relevance (beyond patient mobility) of markers for exercise capacity also in the LVAD population.30
The correlation between FC and survival is well described in HF patients not receiving mechanical circulatory support, but has not been extensively studied in LVAD recipients; hence, the prognostic value of pVO2 has never been reported in this patient group. In contrast, the prognostic value of the 6MWT was elucidated by Hasin et al. in 2012.24 That study included 65 patients, of whom 20 were deemed poor performers (i.e. 6MWD <300 m) postoperatively. Despite similar perioperative HF severity, the poor performers showed poorer survival (i.e. 6MWD <300 m was found to be independently associated with worse survival). 24
Why Does Exercise Capacity Remain Reduced After LVAD Implantation?
Right and Left Ventricular Contractility
Noor et al. showed that in HMII recipients (n=30) 6 months after implantation, pump speed reduction led to significant decline in pVO2 in patients with a left ventricular ejection fraction (LVEF) <40%, but did not alter FC in those with left ventricular (LV) recovery (i.e. LVEF >40%).25 In 2014, in a double-blind crossover study including HMII recipients, Jung et al. showed that increasing pump speed augments pVO2, leading to the conclusion that future generations of continuous-flow LVADs should include a speed change function to improve FC in this patient group.50 In 2018, these findings were confirmed in a study investigating Jarvik 2000 recipients, although that study reported a possible increased risk of AEs (obstructive sleep apnoea).40
However, conflicting data exist, because a recent retrospective study including 49 patients (HeartWare [HW]=6, HMII=43) found that neither right ventricular (RV) nor LV function was associated with the improvement in pVO2.43 In accordance with these data, we recently documented that RV function, even during exercise, was not correlated with pVO2 in LVAD recipients.63 This is in contrast with the results obtained in HF patients not supported by an LVAD. For example, Murninkas et al. found that with every 10% worsening of RV function, pVO2 worsened by 0.97 ml/kg/min.67
The studies described above are small, with considerable heterogeneity, and clearly more studies are needed to establish the importance of intrinsic cardiac contractility in the FC of LVAD recipients.
Chronotropic Incompetence, Arrhythmia and Pacing
Several studies have documented the negative effects of chronotropic incompetence on exercise capacity in LVAD recipients.12,21,27,38,46 Depending on the definition of chronotropic incompetence, it has been reported in approximately half of all examined LVAD recipients. Chronotropic incompetence may represent a somewhat clinically modifiable factor, because many LVAD recipients receive beta-blockers, digoxin or amiodarone and are equipped with pacing devices that could be programmed to improve chronotropic competence. In fact, in a recent study in 30 patients, turning on rate response pacing in LVAD recipients with pacing devices was shown to improve FC (6MWT and treadmill FC) most clearly in patients with chronotropic incompetence.68
Perioperative AF has also been associated with lower FC after LVAD implantation.48 In an analysis from the ELEVATE registry of 194 patients with an HM3, preimplantation AF was an independent predictor of poor performance (6MWD <300 m) 6 months after implantation.48,58
In patients with LVADs, LV preload is of dire importance, and patients with AF (with or without symptoms) lack the atrial kick that could impair RV function, which, in turn, affects the LV preload. Whether pharmacological therapy or AF ablation after LVAD implantation to restore sinus rhythm will improve exercise capacity has not been tested. Future studies are needed to further explore these findings to enable improvements in current technologies.
Pump Design, Placement and Settings
There is no evidence to suggest that one continuous-flow LVAD (e.g. axial versus centrifugal design) is associated with better postimplantation FC than other continuous-flow LVADs. Suboptimal cannula position will lead to reduced circulatory support and would likely impair FC, but this has not been studied in detail.69 Increasing pump speed during exercise has been investigated in several studies and, in most, has been associated with improved FC.36,40,43,50,70 Likely, a future ‘smart pump’ with the ability to increase pump speed in response to increased LV filling during exercise would be beneficial for the FC of LVAD recipients.
Comorbidities
Recently, Schmidt et al. showed that weight gain after implantation is linked to less improvement in FC (specifically, there was a negative correlation between weight gain and absolute pVO2 improvement) and that pVO2 plateaus after implantation with LVADs.45 Regarding weight, recent reports suggest that BMI does not affect patient survival.71–73 However, it could be speculated that weight gain (as seen in Schmidt et al.45) could affect specific AEs; in particular, large body size and associated comorbidities, such as diabetes, may leave the patient more prone to infection, thereby lowering FC, as discussed above.74
In studies investigating blood chemistry, haemoglobin, preoperative C-reactive protein and persistently low perioperative serum albumin concentrations were associated with lower FC after implantation.30,41,44 All these parameters are adjustable. Surprisingly, B-type natriuretic peptide (BNP) was not associated with FC measured as pVO2, although increasing BNP concentrations were associated with a lower QoL.20
Iron deficiency is common in LVAD recipients, but its effect on survival, hospitalisations and FC has not been clearly established.75–77 A recent pilot study of 33 patients was unable to show the expected significant improvement in the 6MWT after intravenous iron replacement 6 months after implantation.78 These findings have yet to be challenged in a randomised prospective study.
Regardless of age, physical training programs (e.g. cardiac rehabilitation) after LVAD implantation have been investigated in several studies, some of which have demonstrated a beneficial effect on FC.21,26,32,37,41 However, others have shown no effect of physical training on FC.13 All studies have shown that physical training is safe and generally well tolerated.14,15,20–23,25,37,38,42–46,49,51–62,80–83
Quality of Life
Overall, both large clinical studies and smaller studies included in this review have reported that QoL improves significantly after LVAD implantation.9,13–18,20,26,49,58
Different studies have assessed QoL using different QoL scores, including the 36-Item Short Form Health Survey (SF-36),13,17,26 KCCQ,9,14,16,18,49 Minnesota Living with Heart Failure (MLWHF) questionnaire9,15,17,18,20,45,49 and the 5-Level EQ-5D (EQ-5D-5L).49,58 There is no consensus as to which QoL score is superior in the LVAD population, and this complicates comparisons between studies. However, data are consistent in showing significant improvements in QoL both in the short and long term (i.e. after a minimum of 6 months follow-up), regardless of methods of quantification.9,13–18,20,26,45,49,58 There is only one exception: a study of 10 LVAD recipients in the early postimplantation stage.45
The mean improvement in KCCQ score was 27 points from approximately 6 weeks to 6 months after implantation.16,49 The greatest increase in KCCQ overall summary score of 178% was seen 24 months after implantation.49 No differences between centrifugal and axial flow pumps have been reported regarding improvements in QoL.49 In two studies that investigated QoL at either 8 weeks or 6 months after implantation, there was a mean change in SF-26 score of 9.8 points versus preimplantion.17,26 The same pattern was seen when investigating QoL using the MLWHF questionnaire and the EQ-5D-5L.9,15,17,18,20,45,49,58
As earlier studies showed that a five-point change in both MLWHF and KCCQ scores is a clinically meaningful change, the improvements described above are highly important.83–85
Some factors related to postimplantation QoL, including exercise rehabilitation in different forms, comorbidities and device characteristics, have been described (Table 1 and Supplementary Material Table 2).13,14,16–18,20 Of these, the most consistent were COPD, diabetes and FC,14,16,20 although conflicting data exist.13 The continued focus on alleviating AEs in LVAD recipients is highlighted by the documented correlation between QoL and AEs.49 Interestingly, one study highlighted the importance of the patient–physician relationship for QoL in LVAD, and it could be speculated that this may be particularly important in patients with AEs.17
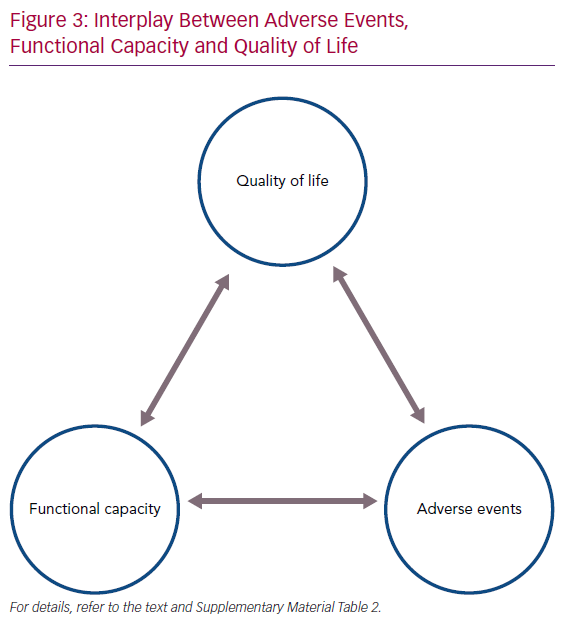
Exercise capacity is closely linked to QoL. Better QoL is related to better muscular strength, treadmill time, anaerobic threshold and pVO2, all of which are factors describing aspects of FC.14,16,26 The fact that increasing FC is associated with better QoL in patients implanted with a continuous-flow LVAD, as in HF patients not supported by an LVAD, highlights the need for continued focus on optimising exercise tolerance.20 Indeed, an interplay between AEs, FC and QoL exists in LVAD recipients, and to improve overall QoL the other two components must be addressed (Figure 3).
Conclusion
Based on a literature review, it is clear that both FC and QoL are severely impaired in advanced HF patients prior to LVAD implantation, but significant improvements are observed after implantation, even though FC remains severely reduced after implantation. Important preoperative predictors of low FC are age, diabetes, COPD, INTERMACS profile, NYHA class, AF and baseline walking distance (e.g. the ability to perform an FC test at baseline). Importantly, poor FC after LVAD implantation is closely related to QoL and is associated with the risk of AEs. These factors should be considered when considering LVAD implantation, especially as destination therapy, and reversible modifiable factors should be aggressively managed both before and after LVAD implantation.
Click here to see Supplementary material.