Heart failure (HF) remains a leading source of morbidity, mortality and economic burden worldwide.1–4 Despite numerous therapeutic breakthroughs provided by landmark clinical trials in stable chronic HF (CHF), there has been little progress over the past two decades in the treatment of acute HF (AHF).5 Therefore, a new paradigm is needed based on better characterisation of AHF for novel clinical discoveries.6–8
Several classifications of HF have been proposed. Based on its temporal course, HF may be classified into CHF and AHF, with latter having two forms: de novo AHF (DNHF), defined as acutely worsened heart function without known underlying heart disease, and acutely decompensated HF (ADCHF), defined as the sudden or gradual onset of symptoms of cardiac failure with known pre-existing cardiomyopathy and a continuum of the natural history of CHF.9–13 Nonetheless, this dichotomisation has only been used for epidemiological purposes.6–8
Interestingly, data extracted from the ASCEND-HF trial provides additional information regarding AHF (DNHF and ADCHF).5 Early diagnosis of HF (≤1 month) before hospitalisation is an independent variable indicating better dyspnoea relief and improved post-hospitalisation mortality in AHF compared with CHF patients. This finding may have an effect on future research regarding treatments and outcomes.5 Thus, the notion of a one-size-fits-all treatment for AHF should be abandoned, and a better understanding of this heterogeneous syndrome in terms of classification is needed. Accordingly, in this review we briefly explain the importance of distinguishing DNHF from ADCHF, which provides a better appreciation of risk factors, prognostication and treatment implications for these two distinct clinical entities.
De Novo Heart Failure Versus Acute Decompensated Chronic Heart Failure
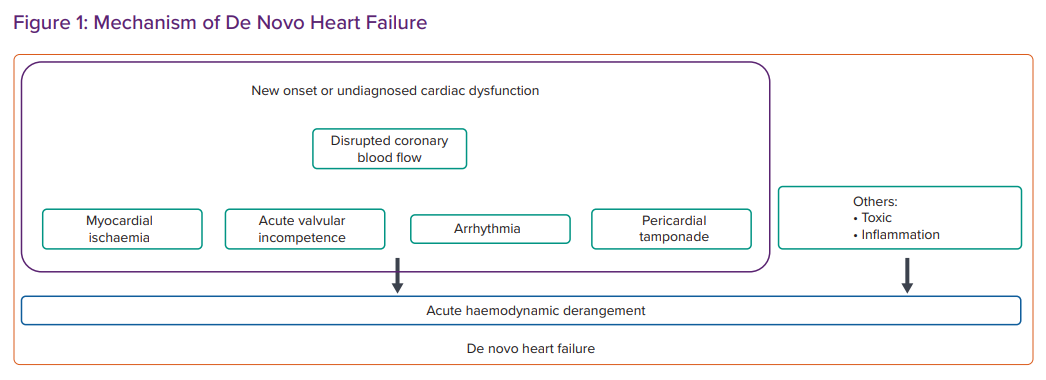
AHF presents as a clinical syndrome. Many classifications have been proposed based on the history of HF, the specific underlying aetiology or precipitating factors, dominant signs and symptoms and major haemodynamic changes, including systolic blood pressure, at presentation (Figure 1).
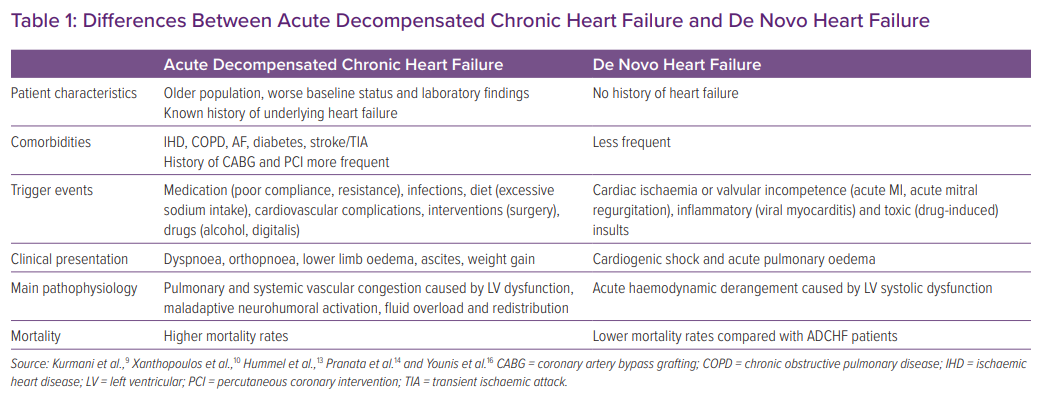
A previous meta-analysis of 15 cohort studies involving 38,320 subjects found that acute coronary syndrome (ACS) and infection were the most common precipitating factors of DNHF and ADCHF, respectively.14 Hypertensive heart disease (HHD) was more frequent in DNHF than in ADCHF. Conversely, comorbidities, such as hypertension, diabetes, ischaemic heart disease, chronic obstructive pulmonary disease, AF and a history of stroke or transient ischaemic attack, were more common in the ADCHF patient group.14,15 Patients with ADCHF also tend to be older and are more likely to have a history of MI, percutaneous coronary intervention, coronary artery bypass grafting and infections.14 Importantly, patients with ADCHF were more likely to be hospitalised in the internal medicine department (Table 1).16
Commensurate with the worse baseline health status of ADCHF patients, laboratory findings revealed lower haemoglobin, higher serum creatinine, lower estimated glomerular filtration rate, higher N-terminal pro B-type natriuretic peptide (NT-proBNP) levels and a Charlson comorbidity index in the ADCHF compared with DNHF population. More importantly, mortality at 3 months and 1 year was significantly lower in the former group.14 A strong association has been found between mortality and chronicity of HF, highlighting the importance of AHF categorisation.16–19
Several studies have demonstrated a possible association between left ventricular ejection fraction (LVEF) and HF outcome.20 A multicentre Korean-based registry reported outcomes for DNHF and ADCHF patients according to LVEF stratification.21 Interestingly, although mortality rates were higher in the DNHF group, there was no difference in mortality rates in the DNHF group among patients with HF with reduced ejection fraction (HFrEF), HF with mid-range ejection fraction (HFmrEF) and HF with preserved ejection fraction (HFpEF). In contrast, in the ADCHF group, HFrEF was associated with higher mortality than HFmrEF and HFpEF.21 The authors of that study postulated that because of the chronic nature of the condition and complication by comorbidities, the ADCHF group did not have a chance to recuperate after acute events.21 In addition, the association between chronicity in HF and long-term mortality is well established, because HF is a progressive disease.
Other notable differences between DNHF and ADCHF are electrocardiographic abnormalities. Based on multicentre European cohorts of AHF, although there was no difference in the prevalence of right bundle branch block (RBBB) between DNHF and ADCHF groups, RBBB was prognostically crucial in DNHF and significantly predicted mortality, even after adjusting for traditional factors.22 In contrast, left bundle branch block and intraventricular conduction delay (IVCD) were more common in the ADCHF group. Nevertheless, only IVCD predicted mortality in the ADCHF group, and this relationship remained significant after adjustment.22
Clinical Characteristics of Acute Decompensated Chronic Heart Failure
As demonstrated in several clinical trials, the natural course of ADCHF is inseparable from that of CHF, but ADCHF and DNHF have different characteristics and outcomes.5,16,21,23–25
There are several notable findings in ADCHF patients: they usually present with signs and symptoms of volume overload and congestion (dyspnoea, orthopnoea, ascites and lower limb oedema) and are associated with higher comorbidity and mortality rates.10 Moreover, ADCHF presents as more profound left ventricular (LV) dysfunction and congestion, as indicated by higher NT-proBNP concentrations, which are associated with worse outcomes.26 Other notable findings in ADCHF patients are pulmonary and systemic vascular congestion associated with a higher end-diastolic pressure–volume relationship.10,27–29
CHF may also cause persistent haemodynamic changes and chronic metabolic derangement, contributing to higher mortality in ADCHF than DNHF.10,14,17 These findings were well demonstrated in a Danish study, in which hospitalisation with CHF was correlated with higher mortality rates and LV dysfunction was a potent predictor of mortality.30
As demonstrated in several studies, patients with ADCHF tend to be older and may have multiple comorbidities, such as diabetes, hypertension, AF, chronic kidney disease and a history of MI. These factors contribute to poorer vital organ function, as reflected by lower serum cholesterol and protein concentrations, higher urea and creatinine concentrations and increased systemic inflammatory markers, which, in turn, contributes to the higher mortality rate.14,28,31,32
Mechanism Underlying ADCHF
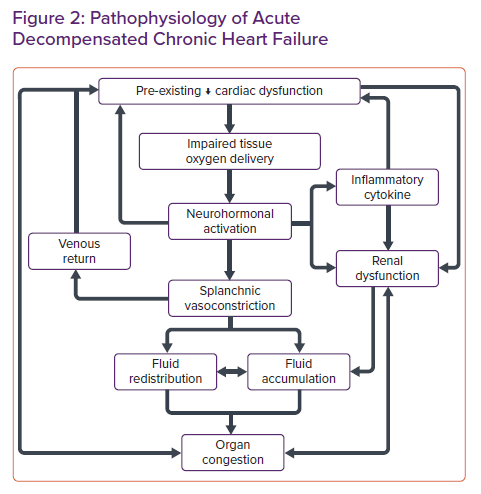
Volume expansion in CHF is a short-term compensatory mechanism to maintain adequate tissue perfusion and is regulated by the neurohormonal response. Nonetheless, this compensatory mechanism becomes maladaptive in the long term.16 Eventually, this altered mechanism leads to fluid accumulation, which results in fluid overload and congestion in the organs. Mitigation of volume overload by diuretics and vasodilators further activates this compensatory mechanism, ultimately leading to further decompensation as the cycle continues.33–35
In addition to fluid accumulation, redistribution of fluid may play an essential role in ADCHF. Sympathetic stimulation caused by dysregulated neurohormonal responses to tissue hypoxia may induce transient vasoconstriction in the splanchnic and peripheral venous circulation, resulting in displacement of fluid into the pulmonary circulation, which contributes to acute episodes of decompensation (Figure 2).36–38
As the cycle continues, a series of decompensation episodes leads to declining function in HF. The exact mechanism behind the deterioration of heart function remains unknown. However, it may be related to the pathophysiology of ADCHF, which consists of two phases, the initiation and amplification phases. The initiation phase is related to several insults that may trigger decompensation of HF, followed by the amplification phase, which induces neurohormonal activation as a host compensatory mechanism for impaired oxygen supply and demand and haemodynamic disturbance. Activation of the inflammatory response may trigger pathological cardiac remodelling, worsening cardiac function, decreasing cardiac output and worsening renal function.39,40
Acute episodes of HF are well known to increase mortality rates, as demonstrated in several studies. The exact mechanism is unknown, but the number of hospitalisations in HF patients is associated with increased mortality rates.41,42 One study hypothesised that as acute decompensation episodes occur, cardiac function will never return to prehospital levels because myocardial damage has occurred.43 This hypothesis has been demonstrated in another study that showed an acceleration in the pathological remodelling of the myocardium, indicated by a transient elevation in troponin I and markers of extracellular matrix turnover (i.e. matrix metalloproteinase 2, tissue matrix metalloproteinase 1 and procollagen type III N-terminal peptides), as the decompensation episode occurs.44
Complex haemodynamic and metabolism changes and maladaptive adrenergic responses play an important role in ADCHF, because these disturbances pertain to the underlying CHF status. Persistent stimulation of β-adrenoceptors in CHF results in the downregulation of these receptors, leading to myocyte contractile dysfunction and increased apoptosis through intrinsic and extrinsic pathways. Modulation of intracellular calcium concentrations, reactive oxygen species (ROS), activation of Fas and tumour necrosis factor (TNF) receptors and initiation of the caspase pathway may result in myocyte apoptosis, direct cardiac dysfunction and decreased cardiac function. Interestingly, altered Ca2+ handling leads to impaired excitation–contraction coupling in the myocyte.43,45–47
Ultimately, LV dysfunction leads to right-sided heart dysfunction, causing severe systemic congestion, including intestinal congestion, major organ dysfunction and adverse metabolic changes that may lead to malnutrition and cachexia and increased mortality.10,48,49
Moreover, impaired oxygen delivery to the peripheral tissue in CHF patients, which is counterbalanced by the maladaptive mechanism described above, causes fluid retention and accumulation in tissues.50,51 Intestinal congestion leads to intestinal oedema, which ultimately impairs nutrient absorption and contributes to malnutrition in CHF patients.17,48 Intestinal congestion, demonstrated by increased gut wall thickness, may contribute to iron deficiency in HF patients that is associated with decreased aerobic performance, increased fatigue and unfavourable outcomes.52–55 A poorly functioning gastrointestinal tract due to oedema, combined with hepcidin dysregulation in HF patients and the intolerable side-effects of oral iron administration, may reduce oral iron uptake.54,56–58 Recent studies show that IV iron administration has a more favourable outcome than oral iron therapy or placebo.59,60 IV ferric carboxymaltose is widely used because of large-scale trials that have been undertaken and have demonstrated the safety of the drug.58 Therefore, current guidelines suggest iron replenishment therapy must be considered in iron-deficient HF patients to improve outcomes, functional status and quality of life.58,61
Intestinal oedema often causes a phenomenon called malnutrition–inflammation complex syndrome, which predisposes to a higher level of circulating endotoxaemia caused by the translocation of lipopolysaccharide from the oedematous intestine, combined with poor nutritional status, which contributes to lower neutralisation and binding of the lipopolysaccharide. The result of this process is a chronic, persistent inflammatory response.31,62
Conversely, obesity paradoxically confers protective effects on CHF patients. Nevertheless, the intricacies of the mechanisms involved have not been fully elucidated as yet, and cannot be explained alone by the cachexia that is caused by HF.63 Renal impairment is a strong predictor of mortality in HF and is more profound in ADCHF than DNHF.64 Several factors may contribute to worsening kidney function, such as kidney hypoperfusion, a higher incidence of chronic kidney disease, renal venous congestion and higher levels of inflammatory cytokines (interleukin [IL]-6) and comorbidities.65–67
From the treatment perspective, diuretic resistance is of concern in ADCHF patients. Although the precise mechanism remains unknown, several factors have been proposed to explain the ineffective response to diuretic therapy. Diuretic resistance may originate from impaired drug absorption resulting from a congested intestine, which consequently reduces the rate of drug absorption and prolongs the time until the therapeutic threshold is reached.68,69 A decrease in kidney blood flow in advanced kidney disease in CHF patients, combined with the use of non-steroidal anti-inflammatory drugs, may further reduce the delivery of diuretics. Endogenous accumulation of anions may compete with the diuretics at their binding sites, resulting in reduced secretion of the diuretic.69 Post-diuretic salt retention is a compensatory mechanism that often occurs after the urinary concentration of sodium is reduced with short-acting diuretics, and, combined with non-compliance with a salt-restricted diet, may negate the effects of diuretics such that a negative sodium balance may not be achieved. The long-term use of loop diuretics may also attenuate their natriuretic effects, as demonstrated in animal studies.69 This mechanism is known as a braking phenomenon and is caused by structural changes in the distal convoluted tubules that lead to increases in sodium reabsorption.69
CHF patients who were hospitalised for non-fatal HF are at the highest risk of dying within 1 month of discharge, with the risk progressively decreasing over time. Interestingly, this is also seen in HF patients hospitalised for other diseases. Furthermore, second and third hospitalisations due to HF confer a 30% cumulative incremental risk of death, which plateaus after four or more hospitalisations.42
Similarly, worsening HF is associated with high early and later mortality, with the risk of the latter being comparable to the risk of death due to MI and stroke.70
The long-term prognosis for ADCHF patients is dismal, with 58% and 48% higher 1- and 10-year mortality risks, respectively, than DNHF patients.16
Together, these findings indicate that although ADCHF patients may survive hospitalisation events due to cardiac or non-cardiac diseases, they may not reach their previous baseline clinical status, which is reflected by their increased mortality. Therefore, key preventive strategies should be identified and implemented to mitigate this excess mortality risk.
Inflammatory Processes in ADCHF
Higher proinflammatory cytokine concentrations are seen in ADCHF compared with DNHF, reflected by higher IL-6 concentrations in the ADCHF group, causing a more pronounced inflammatory response. The profound inflammatory response may be caused by a complex mechanism involving interactions between venous dilatation caused by a rise in right atrial pressure, catecholamine release and β-adrenoceptor activation, resulting in myocardial injury and tissue hypoperfusion.43,71,72
Elevated inflammatory cytokines and responses further exacerbate haemodynamic abnormalities and may directly affect the myocardium through various mechanisms. Intestinal congestion further leads to translocation of endotoxin, also known as lipopolysaccharide, and may induce TNF-α and other inflammatory cytokines. Several effects are associated with increases in TNF-α, such as LV dysfunction and remodelling through the ROS pathway, increased intracellular caspase apoptosis signalling and calcium dysregulation, resulting in impaired contractile proteins in myocytes and the induction of cardiac cell death, as well as the development of anorexia and cachexia, which lead to malnutrition and low protein utilisation.71,73,74 IL-6 is a well-known inflammatory marker, released as an inflammatory response from damaged tissue, which, in this case, is the myocyte. However, an extended sustained release of IL-6 may lead to maladaptive hypertrophy and a decrease myocyte contractile function, which depresses cardiac function.75–77 Other effects seen following the sustained release of IL-6 are endothelial dysfunction, a decreased diuretic response, activation of the neurohumoral response and a reduction in the glomerular filtration rate, which further exacerbates the cardiorenal syndrome, mediating the adverse effects of angiotensin II and promoting sodium retention by activating renal epithelial sodium channels.78–80
Thus, higher inflammatory and metabolic burden caused by a complex interaction between the haemodynamic disturbance, organ dysfunction and fluid accumulation may contribute to the higher mortality in ADCHF than DNHF.17
Clinical Implications: Risk Stratification, Therapeutic Management and Design of Clinical Trials
Currently, the initial therapy for HF is based on a clinical assessment of congestion (wet versus dry) and/or peripheral hypoperfusion (warm versus cold), leading to four classification options. Although most patients with AHF admitted to hospital present with signs and symptoms of congestion, diuretics and vasodilators have been the mainstay of therapy unless systolic blood pressure is <90 mmHg, in which case inotropic agents and vasopressors may be considered.61
Understanding the plethora of differences between DNHF and ADCHF has far-reaching public health and clinical consequences. First, there are differences in risk factors between these two groups, and so different preventive and curative strategies for AHF events should be used. In DNHF, hypertensive heart disease is prevalent. Therefore, health promotion of lifestyle modifications and a healthier diet for hypertensive individuals identified through screening, complemented with optimal guideline-directed medical therapy (GDMT), are crucial for modifying this risk factor.81
A study from Korea showed that, compared with ADCHF patients, DNHF patients had more pronounced prognostic implications of adherence to GDMT.82 Early initiation of angiotensin-converting enzyme inhibitor and angiotensin receptor blocker therapy reduced the rate of rehospitalisation (HR 0.57; 95% CI [0.34–0.95]), mortality (HR 0.41; 95% CI [0.24–0.69]) and the composite endpoint (HR 0.52; 95% CI [0.36–0.77]).82
Moreover, AHF events are precipitated mainly by acute coronary syndrome, specifically ST-elevation MI.14 Thus, mitigating cardiac injury through rapid reperfusion and myocardial protection strategies is crucial in preventing lifelong ventricular dysfunction.83 Indeed, this transient but life-threatening STEMI event was demonstrated in the ASCEND-HF analysis, which showed that 30-day mortality was higher in the DNHF than ADCHF group, although 1- and 10-year mortality was lower in the DNHF group.5
In contrast, ADCHF patients were sicker and complicated with many comorbidities, which rendered them more vulnerable to infection.5 Understandably, infection was the most common precipitator of HF in this group. Respiratory infection accounts for the majority of decompensation in HF patients (>50%).14,84 Thus, it seems rational to prevent infection by vaccination. Indeed, a large Danish cohort study demonstrated that influenza vaccination reduced the risk of all-cause and cardiovascular deaths in HF patients.84 Similarly, pneumococcal vaccination is putatively useful in preventing the precipitation of acute decompensation episodes in ADCHF patients. Nonetheless, investigations with more extensive clinical trials are needed.85
Optimal GDMT is equally important for CHF patients to prevent acute decompensation episodes. CHF is associated with excessive neurohormonal activation and maladaptive compensation, which can be halted by several agents.86 Renin–angiotensin system (RAS) blockers and β-blockers have been shown to improve quality of life and commensurately decrease morbidity and mortality.87 In addition, newer agents can be added to the armamentarium, including angiotensin receptor–neprilysin inhibitor (ARNI) and sodium–glucose cotransporter 2 (SGLT-2) inhibitors, which are oral antidiabetic drugs.88,89
Consequently, managing chronic heart failure patients has become more complex, and this particularly reflected by studies showing that many cardiologists fail to uptitrate drug doses to reach guideline-recommended doses.90
Moreover, in the acute setting (i.e. ADCHF), rapid in-hospital decongestion through IV therapy is the rule to reduce morbidity.91,92
Other than for symptom relief, adequate reduction in congestion is equally important for reducing subsequent hospitalisations.93 When a patient’s haemodynamic status has stabilised, ARNI can be started. In the PIONEER-HF study, ADCHF patients who received ARNI had lower NT-proBNP concentrations than those who received enalapril.94 More importantly, there was no significant difference in side-effect rates between these two agents.
In addition, the distinction between DNHF and ADCHF is essential in terms of risk stratification. The risk stratification for in-hospital mortality of acutely decompensated HF patients has been established in a study using registry-based data, which simplified AHF scenarios into a single entity.95 Thus, further research that prognostically evaluates the in-hospital mortality of AHF subjects would benefit from classifying AHF into two distinct groups.
Finally, to improve the clinical outcomes and quality of care for DNHF and ADCHF patients, future clinical trials should address the underlying differences between the two conditions by providing a dichotomisation between DNHF and ADCHF.14 No real treatment progress for AHF patients over the past several decades may be the result of combining these two different entities.5,49
Indeed, the importance of classification in HF can be seen in previous clinical trials. By classifying CHF on the basis of ejection fraction, pharmacological breakthroughs for this disease can be better appreciated: there are many drugs available to modify the course of HFrEF, leading to direct and indirect improvements in quality of life, morbidity and mortality of CHF patients.89,96–98 In contrast, no meaningful development has been made in the treatment of patients with HFpEF.89,99,100
Similarly, compared with AHF patients with a previous HF diagnosis, a diagnosis of AHF and HF ≤1 month before hospitalisation was independently associated with more significant early dyspnoea relief and improved post-discharge survival.5 Therefore, consideration should be given to distinguishing de novo or recently diagnosed HF from ADCHF when designing future acute HF trials.5
Conclusion
AHF is a cardiovascular emergency syndrome leading to mortality, morbidity and economic burden worldwide. Nevertheless, evidence-based and effective treatment options remain limited. Thus, innovative solutions are sorely needed. Future clinical trials of therapy should address the potentially different phenotypes between DNHF and ADCHF if meaningful discoveries are to be made.