Despite improvements in survival of patients with heart failure (HF) attributed to neurohormonal and device therapies, some patients with advanced HF may still need and benefit from heart transplantation or durable left ventricular assist device (LVAD) support.
LVADs should be considered in patients with INTERMACS profile of 2–4 and in those with INTERMACS profiles of 5–6 who have high-risk characteristics. Patients recovering from INTERMACS level 1 with no irreversible organ damage may also qualify for an LVAD.1
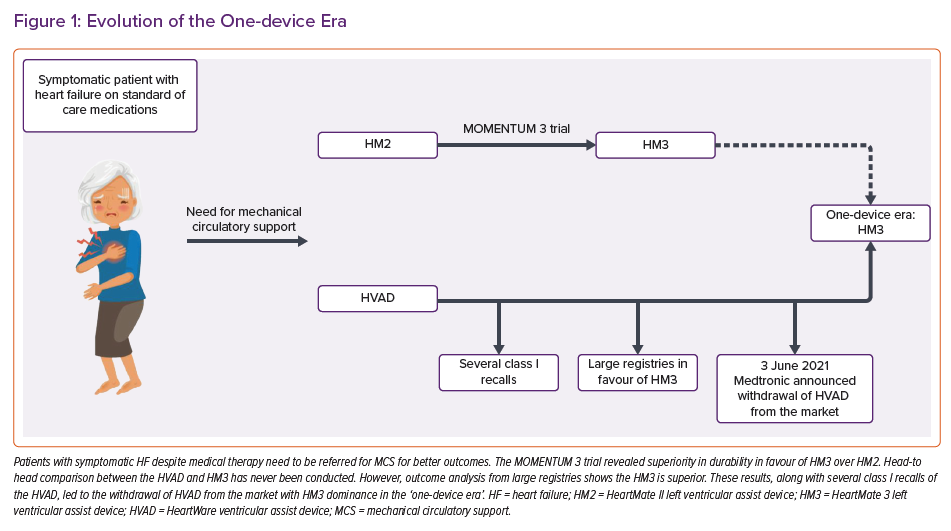
The first generation of LVADs were bulky, pulsatile pumps implanted in the abdominal cavity and had poor durability and high complication rates.2
The second-generation continuous-flow pumps were smaller devices with fewer moving parts, implanted within the thorax and had better safety profiles, with fewer complications and improved durability.3 Among this group, the axial-flow HeartMate II LVAD (HM2) (Abbott), built with mechanical bearings, was associated with a relatively high incidence of pump thrombosis and malfunction.
Among the third-generation LVADs, namely the centrifugal-flow HeartWare ventricular assist device (HVAD) (Medtronic) and HeartMate 3 (HM3) (Abbott) devices, the HM3 demonstrated superior survival, with patients less likely to experience a disabling stroke than those with a second-generation HM2.4–6
Both the HVAD and HM3 had been approved for support in patients with advanced HF as a bridge to transplantation or as destination therapy given their promising outcomes.7 However, on 3 June 2021, Medtronic announced the withdrawal of the HVAD from the market.8 This decision was in light of growing evidence from observational studies showing higher incidences of pump malfunction and neurological adverse events with HVAD than with other devices.
From the unmatched cohorts of the INTERMACS and EUROMACS, it was apparent that the HVAD was implanted more frequently in small patients, women and in patients with lower INTERMACS profiles (those who were sicker).9–11 Whether this was related to device factors will not be determined as a head-to-head comparison between HM3 and HVAD will not be performed.
Moreover, since the approval of HVAD in 2012, it has been subject to 15 class I recalls compared to only two on HM3, in addition to the pump restart failure events, as the company stated.8 Despite the expected income loss and the related expenses, Medtronic has established a programme to support the approximately 4,000 patients who are currently supported with the device.8
Since this abrupt announcement, HM3 has been left as the solitary FDA-approved LVAD in the arena of adult mechanical circulatory support (MCS) (Figure 1). The need for HM3 to be adapted to other patient groups is paramount to fill the gap in the device market created by the HVAD withdrawal and, given this, several changes are expected in the new one-device era.
It should be emphasised that, despite the existence of facilities and therapies, patients with HF are often referred to advanced HF centres too late. Identifying warning signs may allow early referral so advanced support can be offered before end-organ failure develops.1,12–14
The two strategies of early LVAD implantation and medical treatment with LVAD implantation after deterioration are being compared in a prospective trial, Early-VAD (NCT02387112).
Impact on Paediatric Patients
Given the rising survival rates of children with complex congenital heart disease following corrective or palliative surgery and the related increase in the paediatric HF population, there is an increase in the need for heart transplantation and LVAD support for children with advanced HF.
Annually, more than 200 durable devices are implanted in children in North America and more than 40 in Europe.15–16 According to the Fourth Annual Pediatric Interagency Registry for Mechanical Circulatory Support (PEDIMACS) report, between 19 September 2012 and 31 December 2019, 365 young patients (aged 13.2 ± 3.9 years) were implanted with continuous flow assist devices including HVAD, HM2 and HM3.15
HVAD implantation has increased in recent years in small children, since these devices enable a good quality of life and better mobilisation than bulky paracorporeal devices. The FDA has determined a lower limit for body surface area (BSA) of 1.5 m2 for HM3 implantation, with no higher weight cut-off point.
The general consensus is that HM3 is suitable for patients weighing above 30 kg. This consensus along with the abrupt withdrawal of HVAD from the market has left patients weighing <30 kg with only paracorporeal MCS solutions.
Recently, evidence regarding the use of HM3 in children has grown. In one cohort, HM3 was successfully implanted in five children with a median age of 14.5 years (9–16.5 years), median weight of 48 kg (40–75.0 kg), and a median BSA of 1.43 m2 (1.36–1.92 m2).17
Moreover, recent data from the ACTION (Advanced Cardiac Therapies Improving Outcomes Network) registry between December 2017 and September 2019 reported the outcomes of HM3 implantation in 35 young patients.18 In the ACTION registry, 28 (80%) were aged <18 years with a median age of 15.7 years (8.8–47.3 years), had a median weight of 65.7 kg (19.1–114.1 kg) and a median BSA of 1.74 m2 (0.78–2.36 m2), with the majority of recipients (63%) having dilated cardiomyopathy; the survival rate was 97% out to the median of 78 days of follow-up.18
Based on emerging evidence, HM3 can be safely implanted in young adults and children weighing >20 kg. For smaller children (<20 kg), with the absence of other alternatives, the paracorporeal Berlin Heart EXCOR will probably be the main device in use, since it comes in various dimensions and has a target population ranging from newborns to adults.19
Overall, about 50% of children implanted with LVADs are discharged home until heart transplantation or as a bridge to decision.20 Given the expected increase in Berlin Heart EXCOR use among low bodyweight children, management of more LVAD-supported children will be carried out in the inpatient setting since this device is not approved yet (in North America at least) for home support.
The mobile driving unit of the EXCOR enables patients to perform some basic daily activities although with some unavoidable limitations. Under appropriate conditions, following adequate training and education of patients and their supporting family, and under the supervision of an experienced staff able to handle and troubleshoot the complex device, this approach may be adopted for home support.
Need for Innovation
In the upcoming one-device era, device innovation is essential, particularly in the paediatric field.
The Jarvik 2015 assist device (Jarvik) was designed specifically for small patients and is to be evaluated in the PumpKIN trial (NCT02954497). Because of the strict inclusion/exclusion criteria, the PumpKIN trial was unable to enrol all the planned cohort so only compassionate cases of the Jarvik 2015 implantation have been reported.21
In one of these case reports, a 10-month-old female (7.3 kg; BSA 0.4 m2) with left ventricular noncompaction and an INTERMACS profile of 2 after cardiac arrest received the Jarvik 2015. Following a long postoperative course, the young patient underwent successful heart transplantation on postoperative day 93 (pump thrombosis was observed during inspection of the explanted device).21 Another case was a 13-month-old male (10kg; BSA 0.4 m2) with hypoplastic left heart in INTERMACS 1–2 who was excluded from the original PumpKIN trial because he had a mechanical tricuspid valve. The infant underwent Jarvik 2015 implantation followed by a successful heart transplantation on postoperative day 77.21
In the single-device era with the absence of an infant-designated intracorporeal device, these cases emphasise the urgent need for accelerated protocols with less strict inclusion criteria. Meanwhile, it is important that patients implanted with the HVAD are managed in collaboration with clinicians experienced in the HM3, since it is likely that some of these patients will need device exchange.22
Given that only the HM3 is now available on the market, surgeons will need to perform the device exchange operation. Several cases of such an upgrading procedure have been reported (mainly because of device thrombosis or driveline infections) in the lateral thoracotomy approach with good outcomes.23,24
The ‘click-in’ mechanism for pump fixation of the HM3 does not function with the HVAD ring. For an implantation of HM3 in the former HVAD position, a rubber seal is placed around the inflow cannula of the HM3 to bridge the minimal space between HVAD fixation ring and the HM3 device to prevent leakage. The inflow cannula of HM3 is placed into the fixation ring, and the cable tie is tightened around the inflow cannula, while the old outflow graft is clamped and kept in the thorax, and an anastomosis between the remaining outflow graft and the new outflow graft prosthesis of the HM3 is performed.24
Although LVAD exchange can be technically performed off pump without the support of the heart-lung machine, on-pump surgery is still recommended for safety issues.24 HVAD-implanted patients should be considered to be prioritised for heart transplantation as device exchange to HM3 is not a trivial procedure.22
Economic Impact
With HM3 being the only device on the market, Abbott should be able to capture all the lost HVAD sales. Whether this development of ceding the market to Abbott will give the company a greater incentive for investing in technical innovations or not or whether this will result in higher or lower costs will become apparent during the coming years.
HeartMate 3 Haemocompatibility
Although HM3 is associated with low rates of thrombotic events, patients are still prescribed a combination of oral anticoagulation and antiplatelet agents. This combination, along with acquired von Willebrand deficiency, activation of the fibrinolytic system, impaired platelet aggregation and alterations in the blood circulation are all attributed to the continuous flow mechanism of the HM3, increasing the risk of bleeding.25
The most common site of bleeding is the gastrointestinal system secondary to angiodysplasia and arteriovenous malformation.26 A comprehensive approach with a multidisciplinary team including specialists in gastroenterology and cardiology is needed.
Antiplatelet removal and haemocompatibility events with the HeartMate 3 pump are subject to an ongoing randomised, double-blind and placebo-controlled non-inferiority trial (ARIES HM3). This will examine two antithrombotic regimens in HM3: vitamin K antagonist with aspirin (100 mg); versus vitamin K antagonist with placebo, targeting INR levels to within the 2.0–3.0 range.27
With the significantly reduced risk of pump thrombosis in the HM3, the need for full anticoagulation with vitamin K antagonists to INR levels of 2.0–2.5 has been challenged in the MAGENTUM 1 study.28 In this pilot study, lower-intensity anticoagulation targeting an INR level of between 1.5 and 1.9 was found to be safe in the short-term phase of the first 6 months after HM3 LVAD implantation.
Another awaited innovation is the elimination of the external driveline responsible for driveline infections and related complications. The first experience of a fully implantable LVAD (FIVAD) was recently reported.29
LVAD Patients During the Pandemic
COVID-19 continues to present medical, social and economic problems worldwide. Routine, ongoing management of patients with an LVAD is essential during the pandemic but should be carried out in accordance with global and local restrictions depending on the prevalence of the coronavirus infection.30
Physicians should focus on social-isolation effects including depression, non-adherence to medical treatment and the performance of the routine blood tests, particularly coagulation profile.31
Telemonitoring may be a good alternative for specific patients, as it reduces the risks from physical contact. Although the HM3 system has properties for unilateral data transfer from the patient’s home to a medical setting, these properties are disabled because of device security issues. Remote, secure bi-directional communication between patients and LVAD clinics should be arranged so this vulnerable population is not exposed to the risks from attending a healthcare institution’.31,32 A multidisciplinary healthcare team including an LVAD coordinator, nurse and physician should be prepared and trained for this purpose.
Patients should be advised to record vital signs, LVAD parameters, weight, INR values and signs of COVID-19 infection (i.e. fever, cough, myalgia, loss of smell and dyspnoea). The LVAD coordinator should record device parameters, and the patient encouraged to send driveline exit site pictures.
Vaccination is highly recommended for patients with an LVAD since they are at increased risk for morbidity and mortality from COVID-19 infection.33–34
During acute COVID-19 infection, the use of prone-position ventilation for respiratory failure is controversial because of the potential risk of outflow graft and driveline compression, impaired venous return and worsening right ventricular (RV) haemodynamics.35 RV failure secondary to acute respiratory distress syndrome needs to be treated to ensure adequate tissue perfusion. In general, the LVAD speed should target the rightward or neutral interventricular septum, and the optimal haemodynamic goals are central venous pressure of 8–12 mmHg and a cardiac index of 2.2 l/kg/m2 or greater.35
Conclusion
The landscape of mechanical circulatory support will experience major changes following the abrupt withdrawal of HVAD from the market. To fill the gaps, particularly in the paediatric field and with the expected need for HVAD replacement, new surgical techniques and device adaptation will be needed. The new single-device era will, hopefully, encourage scientists and engineers to create innovations and convince politicians for the need for accelerated approval processes in order to meet increasing requirements.