The incidence and prevalence of heart failure (HF) is on the rise due to an ageing population.1 It is estimated that there are around 64.3 million people with HF worldwide.2 In view of more timely diagnosis and more effective management of ischaemic heart disease, hypertension, valvular heart disease and HF itself, the average age of HF cohorts is increasing.3 For a long time, the mainstay of HF medical therapy entailed the titrated use of prognostic medications such as β-blockers, renin–angiotensin–aldosterone system inhibitors and mineralocorticoid receptor antagonists (MRA) in conjunction with a sequential nephron blockade with loop, thiazide and osmotic diuretics for symptomatic control. In recent years, the list of evidence-based HF medications has broadened to include angiotensin receptor–neprilysin inhibitor (ARNI) and sodium-glucose cotransporter 2 inhibitors (SGLT2i). Currently, various international guidelines such as those by the European Society of Cardiology advocate a combined use of the available evidence-based medication at the highest tolerated doses. 4 As such, optimal medical management of HF is becoming increasingly more complex, with the use of between four and five different medications now the norm.4 This is compounded by the additional prescriptions that older people with HF tend to receive for chronic health conditions that commonly coexist with HF, such as hyperlipidaemia, ischaemic heart disease, AF, hypertension, depression, diabetes, chronic kidney disease and chronic obstructive pulmonary disease.
Dumbreck et al. examined NICE guidelines for three exemplar conditions: heart failure, type 2 diabetes and depression.5 The authors compared the treatment recommendations for these conditions with the guidelines for nine other potential chronic health conditions and found that adherence to guidelines for HF in people alongside adherence to guidelines for the other chronic health conditions included in the study resulted in 10 potentially serious drug reactions.5 That paper is seminal in the argument behind the need for prescribing expertise in multimorbidity.
Polypharmacy is broadly defined as the regular use of multiple medications, and it can be divided into minor polypharmacy (two to four drugs), major polypharmacy (five or more drugs) and extreme or hyper-polypharmacy (≥10 drugs).6 There is a positive correlation between increasing age and the likelihood of falling into one of the above categories of polypharmacy, due to both appropriate prescribing for multiple coexisting conditions that accumulate with increasing age and inappropriate prescribing.7 Several tools exist to determine the appropriateness of a prescription, including STOPP (Screening Tool of Older Persons’ Prescriptions) and START (Screening Tool to Alert to Right Treatment), which is validated in the UK, the Beers Criteria and the Medication Appropriateness Index (MAI).8–10 The MAI measures the appropriateness of a prescription for older people by using 10 criteria for each medication. The clinician will then rate each prescription according to the explicit instructions given by the index tool to provide an overall score. Generally, a score of ≥3 indicates that the medication is likely inappropriate. Polypharmacy alone is associated with difficulties taking all recommended medications. The reasons for this include side effects, as well as forgetting timings and the dosage of each medication. Additional downsides of polypharmacy include drug–drug interactions, drug–disease interactions, a higher incidence of adverse effects and drug errors, including both overuse and underuse.11
This narrative review explores the current situation with regard to the HF medical management algorithm and the impact of polypharmacy on the effectiveness and safety of HF therapy. Furthermore, it examines the roles of the geriatrician and pharmacist, as part of a multidisciplinary team (MDT) approach, in minimising potentially inappropriate polypharmacy and their effects on older people with HF.
Latest Guidance on Heart Failure Management
The 2016 European Society of Cardiology (ESC) guidelines for the management of chronic HF recommended a stepwise initiation and uptitration of HF medications tailored to symptom control.12 These recommendations were transformed by the advent of new, large-scale trials. The DAPA-HF and EMPEROR-Reduced trials have clearly demonstrated a reduction in the composite endpoint of mortality and hospitalisation for HF associated with the use of dapagliflozin and empagliflozin, respectively, in patients with chronic HF with reduced ejection fraction (HFrEF), with and without diabetes.13,14 The SOLOIST-WHF trial showed that the use of sotagliflozin, a sodium–glucose cotransporter 1 inhibitor/SGLT2i, was associated with a lower risk of cardiovascular death, hospitalisation for HF and urgent HF visits in people with diabetes with preserved (25% of the cohort) or mildly reduced or reduced ejection fraction (75% of the cohort) following hospitalisation.15 The VICTORIA trial demonstrated a 10% reduction in a composite outcome of death from cardiovascular causes or first hospitalisation for HF with the use of vericiguat, a novel oral soluble guanylate cyclase stimulator.
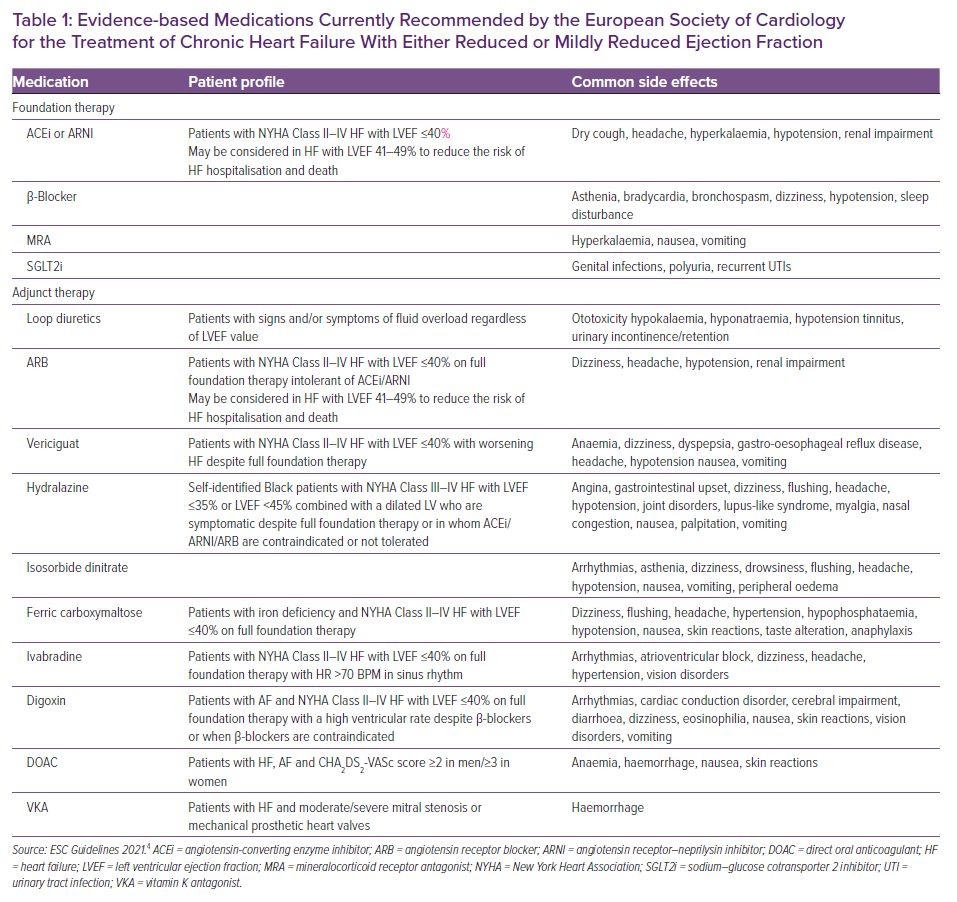
In view of new evidence, the updated 2021 ESC guidelines now recommend the use of a foundation therapy consisting of a combination of an angiotensin-converting enzyme inhibitor (ACEi) or ARNI, β-blocker, MRA and SGLT2i from the beginning (initiated at any visit) in people with HFrEF rather than adding in medications in a stepwise fashion as advised previously.4 The new guidelines also recommend the use of a loop diuretic in people with HFrEF and signs or symptoms of congestion.4 Vericiguat, hydralazine, isosorbide dinitrate, digoxin, ivabradine and intravenous ferric carboxymaltose (Ferrinject) can also be considered as additions to the above list of medications in instances of worsening HF despite the foundation medical therapy in select people with HFrEF (Table 1).
HF with preserved ejection fraction (HFpEF) syndrome is closely intertwined with the ageing processes from a pathophysiological standpoint and, consequently, is often considered a disease of old age. A meta-analysis of large clinical trials in HFpEF and HF with mildly reduced ejection fraction (HFmrEF), such as I-PRESERVE, PEP-CHF, CHARM-Preserved, SENIORS, DIG-Preserved, TOPCAT and PARAGON-HF, showed no evidence of efficacy associated with the use of the foundation therapy medications apart from a potential reduction in the risk of cardiovascular death observed with β-blocker use in people with an ejection fraction of 40–49%.16 Candesartan may have a potential role in people with HFmrEF, as seen in the CHARM Programme, albeit from a post hoc analysis.17 The PARAGON-HF trial showed that sacubitril/valsartan does not improve the risk of HF hospitalisation and cardiovascular death in people with HFpEF.18 However, there was a heterogeneity of treatment effect, with possible benefit seen in prespecified subgroups of patients with left ventricular ejection fraction (LVEF) 45–57% and women.18 In the TOPCAT trial, spironolactone had no significant effect on the composite outcome of death from cardiovascular causes, aborted cardiac arrest or HF-related admissions for HFpEF, but the use of spironolactone led to a significant reduction in HF hospitalisations.19 The latest ESC guidelines still recommend consideration of the use of the foundation therapy in HFpEF and HFmrEF, albeit with a lower quality of evidence (Class IIb).4 The EMPEROR-Preserved trial showed that the SGLT2i empagliflozin reduced HF-related admissions or cardiovascular death compared with placebo in people with HFpEF.20 Many older adults with HFpEF live with polypharmacy, some of whom will experience negative effects. A retrospective analysis of the TOPCAT trial involving 1,761 participants with LVEF ≥45% and a median age of 72 years showed that 37.5% of people were on between five and nine drugs daily, 35.9% were on 10–14 drugs daily, and 19.6% were on ≥15 drugs daily; this left only 7.0% with a low medication burden.21 That study also demonstrated that the three groups with a high medication burden were all associated with a reduction in all-cause mortality, but elevated risks of HF-related and all-cause hospital admission during a 6-year follow-up.
Polypharmacy in Heart Failure
The concept of polypharmacy is well described in the geriatric medicine literature and is becoming more prevalent in HF.22 A study by Goyal et al. showed that at least 75% of ambulatory people with self-reported HF take five medications and at least 25% take 10 medications.23 The medication burden may be even higher among those hospitalised, as demonstrated by the subanalysis of the REGARDS cohort in which, upon discharge, 96% of participants were taking at least five medications and 57% of participants were taking at least 10 medications.24
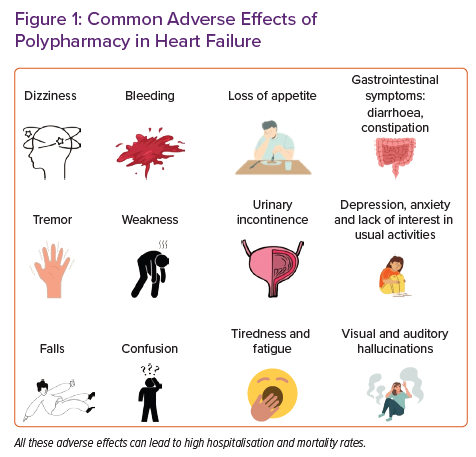
Polypharmacy is associated with a higher rate of adverse drug reactions and a higher treatment burden.25 Treatment burden is the amount of work required from a person for them to participate in their healthcare (e.g. setting alarms to remember to take medications). This can be additive to the quality-of-life impairment brought about by the disease itself.26 Falls, fractures, cognitive impairment and urinary incontinence are very well-recognised adverse effects of polypharmacy. Some of the common adverse effects are shown in Figure 1, whereas Supplementary Table 1 presents some of the common drug–drug interactions in people with HF. In the UK, drug-related adverse events lead to 6.5% of unplanned hospital admissions, thereby taking up 4% of hospital bed capacity due to a median length of stay of 8 days.27 A longitudinal study by Dhalwani et al. found a 21% higher fall rate among those taking five or more medications in adults aged >65 years.28 Approximately 5% of falls among older people result in a fracture, and fall-related injuries are the fifth leading cause of death for older people.29 It is understood that several groups of medications can exert an effect on urinary continence, particularly in older people. For example, calcium channel blockers, diuretics, MRAs and ACEi can all contribute to urinary incontinence, either directly or indirectly.30
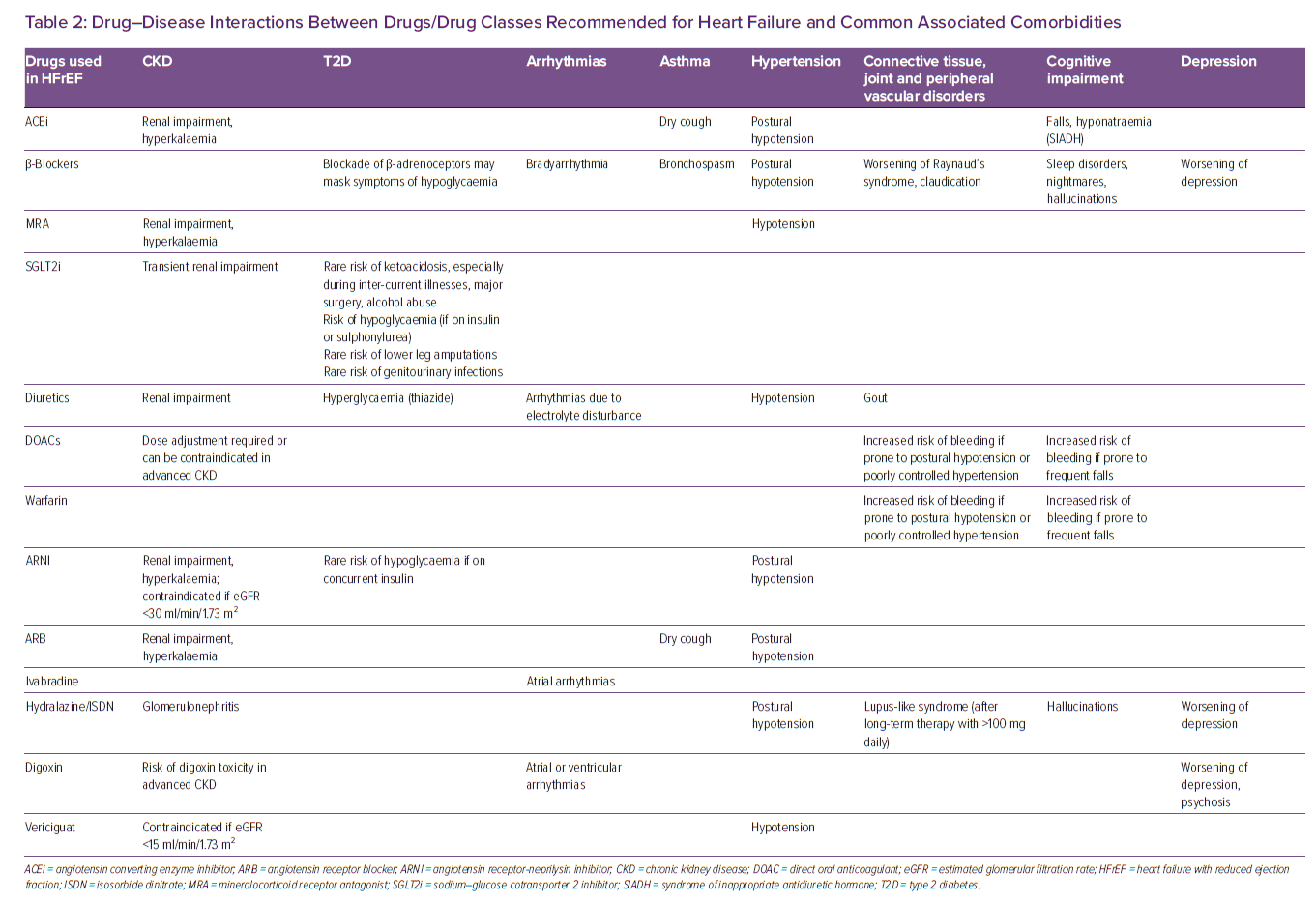
Another significant problem that can arise from polypharmacy is that of cognitive impairment, particularly with the increasing use of anticholinergic medications. Anticholinergic burden is the cumulative effect of taking one or more medications with anticholinergic properties and can be determined using the anticholinergic burden score calculator, which is readily available and user-friendly.31 Anticholinergic burden is a predictor of cognitive impairment in older people and is associated with increased risk of mortality and cardiovascular events, even when accounting for prior cardiovascular comorbidities.32,33 It is imperative that the clinician responsible for optimising heart function is aware of this association. Table 2 presents some of the common HF drug–disease interactions.
Polypharmacy is also known to be associated with higher risks of readmission.34 HF has the highest rate of 30-day readmission compared to acute MI and pneumonia, which may be explained, in part, by a high prevalence of polypharmacy in this population.35,36 Most people with HF are aged >75 years; this group not only has a higher level of morbidity, but also more barriers, both personal and service-derived, that limit optimal care. As such, older people with HF require a different clinical approach and outlook when it comes to making treatment decisions.
Paradoxically, polypharmacy is associated with the underutilisation of guideline-directed medical therapy in HF.37 It appears that with an increasing number of medications on a prescription chart, clinicians are sometimes reticent to initiate appropriate medications for fear of contributing to the ever-growing list. It has also been shown that people may struggle to adhere to treatment regimens when they are more complicated (e.g. an increasing number of prescriptions or an increasing frequency of dosing, particularly if drugs are advised to be taken more often than twice per day or are to be taken outside of the standard ‘morning’ and ‘night’ times).38–40
Large cardiovascular societies are recognising the need for active deprescribing and promote consideration of medication withdrawal or dosage reduction to correct or prevent medication-related complications.41,42 The fundamental principle of prescribing medications only when the benefit outweighs the risk has, in recent years, been complemented by the paradigm shift towards active deprescribing when harms outweigh benefits. Deprescribing is defined as ‘medication withdrawal’ and is usually done with the intention of improving outcomes that are important to people.43 The key characteristics of deprescribing are that it is comprehensive, systematic and proactive, aiming to identify potential future problems and avoiding them if possible. Deprescribing cardiovascular medication can be a complicated and often more time-consuming process than prescribing medication in the first place. Although multiple international cardiovascular societies acknowledge the role of deprescribing in older adults, there are no clear guidelines on when and how to perform it. There are various barriers to deprescribing, such as a lack of clear scientific evidence, the design of the health system and physician- and patient-derived barriers.44 This reflects the broader culture of medicine, where the process of disease management implies the need for pharmacotherapy.45
The medications that may cause or exacerbate HF are very common and could be considered as the first-choice targets for deprescribing. Non-steroidal anti-inflammatory drugs, metformin, cyclizine, dipeptidyl peptidase-4 inhibitors, calcium channel blockers, doxazosin, tamsulosin and certain antiarrhythmic, antiepileptic and antiparkinsonian drugs are among the most common medications prescribed in older people; these drugs have the potential to worsen HF.46 A subanalysis of the REGARDS cohort showed that 41% of people aged ≥65 years who were hospitalised with HF were, on admission, taking at least one medication that could cause or exacerbate HF; upon discharge, this was true for 37% of people.47 In the same study, 21% of these people, upon discharge, were taking at least the same number of HF-exacerbating medications as they were on admission, with some taking more.47 The Beers Criteria outline potentially inappropriate medications for older adults where risks outweigh the benefits.9 The subanalysis of the REGARDS cohort showed that 61% of older adults admitted with HF were taking medications deemed inappropriate by Beers Criteria on admission to hospital, and this was the case for 60% of older adults at the point of discharge.47
When making decisions about medication initiation or continuation in older adults, it is worth bearing in mind that most available evidence, and consequently most guidelines, may not necessarily apply to older adults because, until recently, most hospitalised older people did not meet the criteria for enrolment in major clinical trials.48 The PREDICT study reviewed the protocols of 251 clinical trials and demonstrated that multiple key HFrEF trials, such as SOLVD, MERIT-HF and RALES, systematically excluded older adults on the basis of multimorbidity (80% of trials), upper age limit and reduced life expectancy (25–35% of trials), cognitive impairment (13% of trials) and polypharmacy (5% of trials).49 Some meta-analyses suggest that older adults may derive benefit from the treatments recommended by current guidelines, but there is a need for more randomised trial data for people with cognitive impairment and chronic health conditions to confirm the efficacy and safety of conventional HFrEF therapies.50,51 The median age of participants in contemporary trials is beginning to increase, the upper age limit has largely been removed and new studies are starting to incorporate person-reported outcomes.52 Hopefully this will mean that more real-world older people with HFrEF will be represented in future work.
The American College of Cardiology outlines the importance of making medication decisions with a holistic approach, taking into consideration that older adults with HF often contend with physical and functional deficits that span multiple domains. The four key domains to consider in this context are:
- the medical domain, which encompasses the HF stage and aetiology, coexisting chronic health conditions, nutritional status and challenges posed by the pharmacological treatment of HF;
- the cognition and emotional health domain;
- the physical function domain; and
- the socioeconomic and environmental domain.53
The latest ESC guidelines on the management of HF endorse the idea of reassessing the appropriateness of prescribed medication in the context of acute decompensated HF admissions and haemodynamic instability.4 Moreover, they advise the cautious use of digoxin in older people. However, they do not explicitly address the issue of polypharmacy.4 Age alone should not be a barrier to the initiation of treatment; however, when there is evidence of a person’s deficits spanning the domains listed above, it would be wise to consider that strict adherence to guideline-directed medical therapy may be inappropriate.
One multicentre parallel group trial looked at the effects of discontinuing statin therapy in the setting of advanced life-limiting illness and demonstrated non-inferiority in survival probability at 60 days when therapy was discontinued.54 In addition, the OPTIMISE trial looked at the dose reduction of antihypertensive medication in older people and demonstrated non-inferiority in systolic blood pressure control (measured at 12 weeks) when therapy was discontinued.55 However, both these trials were relatively small with short follow-up periods, and there is therefore still a lack of robust evidence-based advice regarding the safety, optimal mode and efficacy of deprescribing to improve person-reported outcomes and cardiovascular events. A study looking at the attitudes of older people with HFpEF found that people had a lot of uncertainty and conflicting attitudes, such as fear of deterioration upon discontinuation of medications, but, at the same time, a dislike of medications.56 A physician survey involving geriatricians and cardiovascular and general internal medicine specialists identified that the two most significant impediments to deprescribing were the reluctance of a patient and a worry that deprescribing may be interfering with another clinician’s treatment plan (regardless of the speciality of each clinician).57 However, in the same study, patients indicated that, with specific regard to cardiovascular medications, they would be willing to temporarily stop medication and observe for any effect, but would want to have the option to recommence therapy if they experienced worsening symptoms.58 This suggests that there may be a need for n-of-1 studies to systematically assess the effect of being on a medication, discontinuing, and then recommencing the same medication. An ongoing n-of-1 trial at Weill Cornell is looking at facilitating shared decision-making regarding β-blocker use in older adults with HFpEF (NCT04757584). This will expand knowledge on deprescribing and help further assess the utility of n-of-1 trials in this context.
Role of the Geriatrician
Some geriatricians and cardiologists with a special interest in medicine for older people advocate the use of conceptual frameworks that reflect the barriers to deprescribing and facilitate interventions to overcome these barriers. Shared decision-making is one such important concept; a study looking at older adults with HFpEF showed that 91% of those surveyed wanted to be involved in decision-making regarding any alteration in their medication regime.59 For clinicians, despite the known utility of shared decision-making, the current structure of the healthcare system does not facilitate such extensive and detailed discussions, with most conversations limited to a few minutes on an inpatient ward round or a 10- to 15-min appointment with a general practitioner. Depending on the availability of beds in the cardiology ward, older people with HF may also be admitted to either a general medical ward or a care of the older person ward rather than a cardiology or HF specialist unit, which means that geriatricians and general medical practitioners are often at the core of delivering cardiovascular care for this group of people.60 As such, despite the stresses and time pressures placed on hospital-based clinicians, and notwithstanding the importance of a proactive primary care physician, it could be argued that geriatricians are well placed to conduct a thorough medication review on older people with HF admitted to hospital at some point during the inpatient stay. However, this is a task that ought to be conducted by all clinicians and not limited to geriatricians.
Recently, there has been a lot of discussion about the role of geriatricians in delivering cardiovascular care adapted to the unique needs of older adults. Geriatricians often work closely with the physicians on the ‘acute take’, which can provide the opportunity for early case identification of people with HF and offer valuable input without unnecessary delay. In addition, critical appraisal of the utility of certain cardiovascular investigations, as well as adaptation of treatment recommendations to better suit the ageing population with HF, has been encouraged.61 Still, it has been argued that geriatricians ought to be vigilant towards and willing to challenge any referral bias to and acceptance by specialist HF services.62 The responsibility of ensuring equity of access to gold-standard investigations and evidence-based management, both pharmacotherapy and medical devices, often falls on the shoulders of the geriatricians looking after older people. Geriatricians, with their extensive broad-based training and experience in caring for people with consideration of more than just what is prognostically best for one organ or system, are best equipped to navigate the minefield of medical management of HF, people’s preferences and conditions of frailty.
Role of the Pharmacist
Polypharmacy intervention tools, such as STOPP/START and Beers Criteria, both developed by geriatricians, and the MAI, developed by a clinical pharmacist, have become popular and credible. These tools have been endorsed by several European societies, including the National Institute for Health and Clinical Excellence (NICE) and the UK Royal College of General Practitioners.63
Pharmacists have been taking an active role in developing and implementing person-centred approaches to medication optimisation, such as introducing pharmacist-led medication reviews, enabling the timely identification and resolution of medication errors and interactions, as well as empowering people to make informed decisions about their treatment with the support of a medication specialist. A medication review aims to strike a good balance between the benefits of pharmacological therapy and the risks of polypharmacy.
A large proportion of polypharmacy intervention is delivered in the community or at hospital pharmacies, by pharmacists alone or in collaboration with a clinician. Pharmacists commonly perform prescription reviews addressing issues such as duplicate scripts, possible drug–drug interactions, non-optimal route and mode of delivery, adherence, compliance and exploring challenges relating to medicine-taking behaviour. However, it is important to note that despite so many interventions, the evidence base for interventions that improve prescribing remains weak. Similarly, there is a lack of evidence to demonstrate a clinically significant effect from deprescribing interventions, as demonstrated in the review by Rankin et al.64 This may be due to siloed working, and perhaps an MDT approach may lead to more success. A pharmacist’s review could be complemented by input from a physician, perhaps a geriatrician, who could perform a clinical medication review assessing drug–disease interactions and adherence to guidelines. In addition, there is a role for clinical pharmacologists, who have a detailed understanding of drug indications across a broad range of diseases because they are dual-trained in general medicine. Clinical pharmacologists also study clinical manifestations of adverse drug reactions, drug–drug interactions and drug–disease interactions. Alongside the geriatrician, they may be best placed to examine risk versus benefit in the case of overlapping diseases.
What matters to a person is key. The end goals of care should be ascertained from each person and considered when making prescribing or deprescribing decisions. Eliciting the priorities of the person receiving care should be pivotal; improved survival may not necessarily be the most pertinent outcome for older people, and living independently is often at the forefront.65,66 Specialists who work closely with older adults, such as primary care physicians, geriatricians and cardiologists, as well as pharmacologists and pharmacists (in both hospital and community settings) may improve the quality of care delivered by exploring people’s wishes and expectations from treatment and explicitly documenting these wishes in care records for other healthcare providers to look back on and guide future management decisions. For many older people, longevity may not be their main or only priority. The clinicians should apply the evidence and guidelines with this in mind, and explore people’s wishes and expectations. By and large, people do wish to be involved in such decision-making about their health, and this should be facilitated by clinicians. There are widespread system flaws that make this difficult to regularly put into practice but, as health experts, clinicians should create an environment to engage in useful discussions with people. Medication reviews are likely to generally involve a pharmacist and, in complex cases, a consultant pharmacologist and geriatrician who can assess the person from a perspective that is not too focused on the health of one organ system alone. Such medication reviews could be strategically planned because they often are within the community, but they could also be performed opportunistically; HF patients are most commonly admitted to non-specialist wards and, often, are admitted to hospital for non-HF-related problems, particularly in th eir last year of life.67 These admissions are opportunities for pharmacists, pharmacologists and all clinicians, not solely geriatricians, to perform a thorough medication review at some point prior to the person’s discharge. Ideally, however, this should be performed as a joint pharmacist–physician enterprise because purely pharmacist-led and purely physician-led interventions have so far failed to show significant impacts. Therefore, it has been recommended that geriatricians and pharmacists be included as a part of the HF MDT, along with other specialists.68 The Liverpool integrated multispeciality multimorbidity HF MDT model started in January 2020 and integrates a community HF team with secondary and tertiary care HF teams, and includes a geriatrician, pharmacist, consultant pharmacologist, renal physician, diabetes physician, chest physician and palliative care physician. This model has been shown to be beneficial in reducing clinic attendance as well as all-cause hospitalisations.69
Conclusion
HF is a common health condition in older people. Older people often have comorbidities, most of which generally require pharmacological therapies. As the number of cardiovascular clinical trials continues to increase, the number of medications available for the management of HF continues to grow. Notwithstanding the importance and proven benefits of such drugs, our attraction to algorithm-driven medicine, whereby a person ought to be prescribed every drug for which they fit the inclusion criteria, does bring some disadvantages, particularly in those who have multimorbidity, which may have conflicting demands. We have seen how polypharmacy due to multimorbidity can reduce compliance in some people and, in certain instances, render some clinicians reticent to prescribe what may be an appropriate medication. It is crucial that clinicians in general, but particularly those who care for complex older people, are aware of the limitations of guidelines and the importance of a broad-based medical education that provides doctors with the ability to assess the risks and benefits of proposed treatment regimens, interpret clinical research and assess applicability to the individual older person in front of them. This review highlights the importance of taking a holistic approach towards HF management, particularly in people with multimorbidity and polypharmacy, through the incorporation of a geriatrician and a pharmacist (among other specialists) in the MDT.