The recent COVID-19 pandemic has highlighted the need for more timely, dependable and remote access to medical care. Consequently, the pandemic has dramatically shifted the approach to patient care on multiple levels. This transformation includes a rapid transition to a virtual physician–patient encounter environment (telehealth) and an increasing investment in remote patient monitoring technology.1 With increasing implementation and experience in remote patient monitoring (RPM), challenges have become more apparent when addressing the needs of vulnerable populations such as women and African-Americans. These challenges include applicability across the broad spectrum of heart failure (HF) phenotypes, disadvantages for those with limited access to the internet, affordability due to frequent non-coverage by payers and poor uptake by patients who are less proficient with technology overall.2 These challenges may represent barriers to the uniform application of RPM and may inadvertently perpetuate disparities in disadvantaged populations.
From risk factors to different pathophysiological processes and clinical phenotypes in HF, sex differences have been well documented.3,4 Traditional risk factors such as hypertension, diabetes, obesity and smoking confer a comparatively higher risk for HF in female patients.5,6 In addition, female patients are subject to sex-specific risk factors such as autoimmune disease, breast cancer therapy, pregnancy and coronary syndromes without atherosclerotic disease.3 Furthermore, they frequently present with pronounced symptoms and generally later in their HF trajectory.7,8 Plasma concentrations of HF biomarkers differ between sexes, which is only partially explained by differences in hormone status.9 As in sex-based differences in the cardiovascular research field, racial disparities have also been described. For example, African-American patients are more likely to die from HF compared with white patients, particularly in the younger age groups (35–64 years) and regardless of sex.6,10 In addition, compared with men and white patients, African-American female patients are less likely to receive appropriate medical therapy, ICDs or cardiac resynchronisation therapy with a defibrillator, or be included in clinical trials.11–14
Whether founded in biology or other factors, these differences may lead to inequities and disparities related to sex and race. While some of these factors are modifiable through better trial design, others require investment in research infrastructure and communities. Here, we review disparities related to sex and race in remote monitoring, elucidate aetiologies that contribute to inequities in black patients and suggest potential ways to mitigate them.
Enrolment of Women and Black Patients in Remote Monitoring Trials
Remote Haemodynamic Monitoring
Small pulmonary artery (PA) pressure monitoring has gained significant importance in managing patients with HF. The only PA pressure monitor (CardioMEMS Heart Sensor; Abbott) currently approved by the Food and Drug Administration has recently received expanded indication for patients with HF and New York Heart Association (NYHA) class II–III symptoms. Most studies that have evaluated the effectiveness of PA pressure monitoring, such as the CHAMPION (CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes) randomised trial, the observational CardioMEMS post-approval study (PAS) and MEMS-HF, have enrolled only a modest proportion of women and black patients.15–17 There was no evidence of effect modification of PA pressure monitoring by sex or race, with results following consistently the overall decrease in HF hospitalisations observed in all studies.18 Subgroup analyses on race or ethnicity were not reported in MEMS-HF. The percentages of women and black patients enrolled are listed in Table 1.
Published in 2021, the GUIDE-HF randomised trial assessed the composite of all-cause mortality and HF-related events in 1,000 patients with NYHA class II–IV symptoms.19 The study results were significantly affected by changing event rates during the COVID-19 pandemic. The two groups (remote monitoring vs. conventional treatment) did not differ in clinical outcomes during the study period; however, a pre-COVID analysis demonstrated significant benefit in the treatment group (HR 0.81; 95% CI [0.66–1.00]; p=0.049) primarily due to reductions in hospitalisations (Table 1). In contrast to CHAMPION, more participants were women (38%). Racial diversity was modest but comparable to the general population of 11–13%; only 17% of enrolled patients were black, while white patients comprised the majority (81%).20 Nevertheless, prespecified subgroup analysis indicated significant interactions for sex (p for interaction=0.01) and race (p for interaction=0.095; level of significance at 0.15), suggesting a more substantial treatment effect of remote PA pressure-guided management in women (HR 0.64; 95% CI [0.47–0.87]) and black patients (HR 0.68; 95% CI [0.48–0.97]) compared with men and non-black patients, respectively. No such finding has been previously demonstrated in similar studies. It has been suggested that such differences may represent treatment bias (implicit bias) and the possibility that these two populations are disproportionately affected by HF, present later in disease progression, and thus are more likely to experience significant benefits with PA pressure-guided treatment. The findings of the subgroup analyses are both hypothesis-generating and encouraging. They could provide the foundation for mitigating disparities in HF treatment and give an outline for future intervention targets. In broader terms, through a protocol-driven clinic intervention and the absence of direct patient–clinician interaction, RPM could facilitate a form of ‘community single blinding’ outside of the clinical trials context that could reduce bias.
Left atrial (HOMEOSTASIS, LAPTOP-HF) and right ventricular (COMPASS-HF) pressure monitoring trials have evaluated the relationship between haemodynamic monitoring and HF-related outcomes.15,21–23 Female participation ranged from 22% to 35%. No information was provided regarding racial or ethnic diversity. While HOMEOSTASIS and COMPASS-HF failed to show significant effectiveness in the overall population, LAPTOP-HF was prematurely discontinued due to perceived excess in procedure-related complications. None of these devices has been commercially available for use.
Cardiac Implantable Electronic Devices
Although landmark trials have demonstrated that ICDs reduce the risk of sudden death in HF with reduced ejection fraction (HFrEF), women were vastly underrepresented.24 Similar observation exists for black patients who are less likely to receive an ICD or cardiac resynchronisation therapy (CRT) despite a clinical indication.13 Consequently, controversy remains regarding the effectiveness of ICDs as primary prevention in women, with one meta-analysis (in which only 19.7% of the enrolled patients were female) that combined five landmark primary prevention ICD trials (DEFINITE, SCD-HeFT, DINAMIT, MUSTT and MADIT II) notably suggesting a lack of significant survival benefit for women randomised to ICD (HR 1.01; 95% CI [0.76–1.33]) while there was a 22% reduction in mortality for men (HR 0.78; 95% CI [0.70–0.87]).25 Additional studies suggest significant under-utilisation of CRT in female patients and black patients, with these disparities increasing over time in the US despite an observed benefit of CRT in the female population.6,26–29 Although the primary goal of these studies was not to evaluate implantable remote monitoring technology, differences seen in cardiac implantable electronic device (CIED)-related remote monitoring may be attributable to the lack of representative inclusion of women and black patients in these initial studies.
The rising prevalence of CIEDs in patients with HFrEF has spurred the evolution of the technology, providing an array of diagnostic measures and leveraging proprietary algorithms that may be used to guide the management of HF in a more individualised manner. Different CIED manufacturers provide variable single or multiple physiologic parameter monitoring, collectively known as HF diagnostics. The sensitivity and effectiveness of intrathoracic impendence monitoring (CorVue; Abbott) were evaluated in the DEFEAT-PE and LIMIT-CHF studies (Table 2).30,31 There was no observed difference by sex or race in the reduction of emergency treatment of HF. Three different studies, TRUST, ECOST and IN-TIME, evaluated the Biotronik Home Monitoring System in reducing all-cause mortality and hospitalisation for worsening HF.32,33 Pooled data from these studies have shown a reduction in all-cause mortality and the composite of all-cause mortality or worsening HF hospitalisations.34 Finally, the DOT-HF and PARTNERS HF trials evaluated the use of intrathoracic impedance in combination with other parameters in Medtronic ICDs and CRTs in reducing HF events.35,36 Although a positive combined HF diagnostics algorithm conferred a higher risk of future HF hospitalisation, it did not reduce all-cause mortality or HF hospitalisations.
Two studies have evaluated the sensitivity of integrated multiparameter algorithms using device diagnostics for future HF events in intermediate to high-risk patients: MultiSENSE and SELENE-HF.37 The MultiSENSE-derived algorithm had a sensitivity of 70% for HF-related events (HFEs) at a nominal threshold of 16, paired with a low unexplained alert rate and a median lead time of 34 days. Alternatively, the proprietary algorithm derived and validated in SELENE-HF had a sensitivity of 66% for predicting the primary endpoint of HF hospitalisation (95% CI: [45.7–82.1%]) with a median alert lead time of 42 days.38 Neither of the two trials described sex- or race-specific interactions (Table 2). Accordingly, the generalisability of the results of these studies, as it pertains to female patients and black patients, remains unclear. In addition, European studies systematically underreport the racial/ethnic background of patients enrolled.
Telemonitoring and Wearable Devices
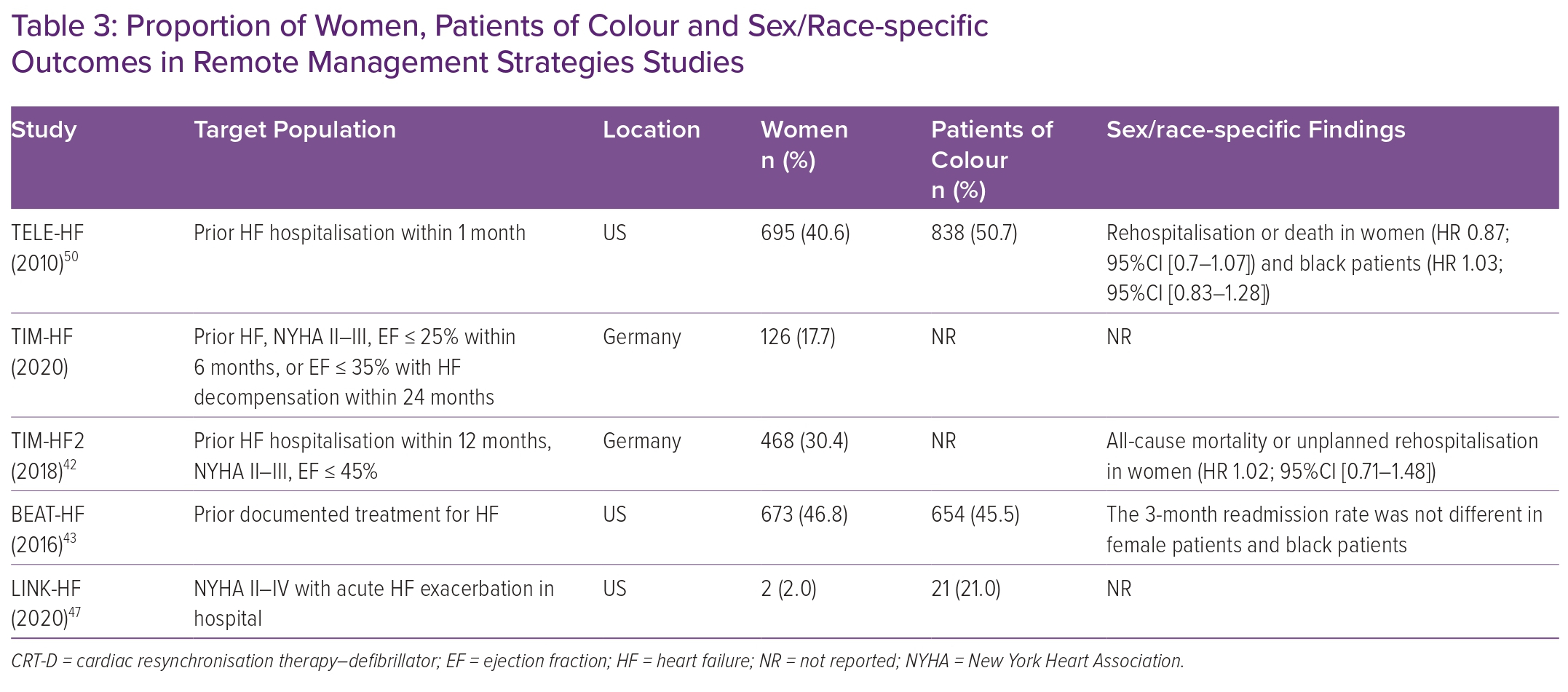
Telemonitoring, unlike RPM, generally uses a multifaceted monitoring approach consisting of patient-derived data from self-reported symptoms, external devices (weight, electrocardiogram, oxygen saturation), bloodwork and more involvement of the patient’s primary clinician to improve HF outcomes.39 Compared with RPM, telemonitoring is dependent on the active engagement and participation of patients to collect data. Several randomised clinical trials have evaluated this approach of remote HF management, including TELE-HF, TIM-HF, TIM-HF2 and BEAT-HF, using a combination of strategies (automated phone systems) or integrated tools (a three-lead ECG, a blood pressure device and a weighing scale).40–43 Enrolment of female patients and black patients was significantly higher than in other remote monitoring studies (Table 3). Of those studies, only TIM-HF2 successfully demonstrated the benefit of remote monitoring in reducing HF hospitalisation days and all-cause mortality (4.88 versus 6.64%; HR 0.80; 95% CI [0.65–1.00]; p=0.046). Nevertheless, this benefit was driven by a primary outcome reduction in men (HR 0.72; 95% CI [0.56–0.95]), while no benefit was observed in women (HR 1.02; 95% CI [0.71–1.49]).
Expanding on wearable technologies for remote monitoring, commercial wearables are used for tracking activity, sleep, ECG, heart rate, oxygen saturation and so on. These devices are increasingly prevalent, especially in younger populations. Still, their accuracy (peak VO2, 6-minute walk test) or effectiveness in improving health outcomes has not been well-studied in large-scale trials and is currently under investigation in the HF population.44 The Apple Heart Study was a large study evaluating the efficacy of the Apple Watch in detecting AF in healthy participants.45 Patients with notification of AF by the watch underwent further testing with an ECG patch. A positive predictive value of 0.84 for observing AF on the ECG was shown for patients notified of an irregular pulse by the watch. Of the total study population of 419,297 participants, 42% were women, but only 12% and 7.7% identified as Latino or black, respectively. Notably, the percentage of women who received a notification for abnormal heart rhythm was almost half of that observed in enrolled men, suggesting a difference in either occurrence or detection of AF in women compared with men (21% versus 77%, respectively). However, real-world data show that although AF is more prevalent in men, the difference seen in the Apple study notifications between women and men suggests possible under-detection in women.46
The LINK-HF study examined the effectiveness of a personalised analytical platform using longitudinal data integrating vital signs to predict future HF hospitalisations via a disposable multisensor chest patch in the Veteran Administration (VA) population (Table 3).47 This was one of the first studies of wearable devices to use machine learning to predict clinical HF deterioration. Of the 100 individuals enrolled, female participants constituted a minute 2% of the entire population. Despite the encouraging findings of the study showing a 76–88% sensitivity in detecting precursors of clinical deterioration, the small sample size limits the generalisability of these findings. Other devices have focused on surrogate measures of pulmonary oedema such as thoracic impedance. In the IMPEDANCE-HF study, which recruited 256 patients (15% female, race not reported) with chronic HFrEF and a recent hospitalisation, and randomly assigned patients to receive standard care or standard care plus monitoring with the Edema Guard Monitor (RS Medical Monitoring), monthly assessments of lung impedance resulted in a 55% reduction in HF hospitalisation, 48% reduction in all-cause mortality and 70% in HF-related deaths.48
Finally, important lessons were learned during the COVID-19 pandemic that forced health systems and individual health providers to offer video and telephone telehealth visits to complement in-person visits. Although this strategy has provided significant benefits for patient health safety, it may have exacerbated health inequities as they pertain to digital health literacy, language proficiency, access as well as proficiency with internet technology, and low socioeconomic status.49
Female patients were 30% less likely to complete a cardiovascular telehealth visit in a recent retrospective study of 2,940 patients in a large urban academic centre.50 Black patients were 17% more likely to achieve a telehealth visit than white patients, although they were more likely to use their telephone instead of a video call.50 In addition, younger patients, patients with commercial insurance, higher income and access to broadband internet were more likely to complete a video telehealth visit, as shown in three recent studies (Supplementary Material Table 1).51,52 Overall, persistent gaps are observed in randomised trials and the community as it relates to telemedicine care in female patients and black patients.
Barriers to Remote Patient Monitoring for Women
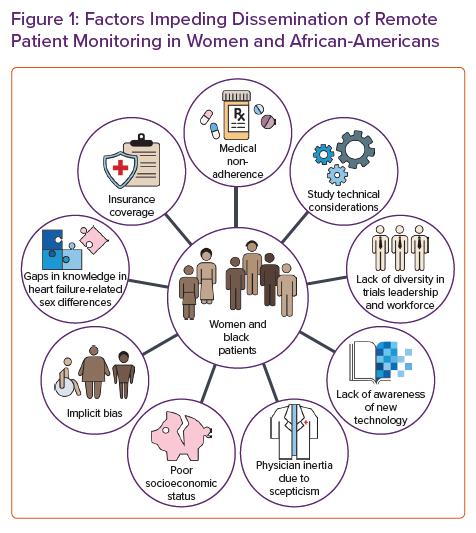
A multitude of causes could explain the persistent gaps in sex-specific research (Figure 1). The lack of sex and racial and/or ethnic diversity in principal investigators and local research staff may discourage women from participating.53 Similarly, sex-specific inclusion criteria, intervention trials (drug, device and surgery), and the location of trial coordinating sites may lead to under-enrolment.53 In addition, female patients may be more risk-averse than men under stress, which could be pertinent in healthcare decisions.54
Despite these well-described contributors, the US government authorised the National Institutes of Health (NIH) Revitalization Act in 1993 to increase the enrolment of women. However, no specific goal has been mandated.55 The limited funding for cardiovascular trials may indirectly limit the conduct of sex-specific studies and indirectly affect the care of women.
Other less tangible causes for the low rate of female participation in trials may pertain to the lack of awareness of available cardiovascular studies among women, traditional sex-specific barriers, such as caregiving responsibilities and long work hours, and decreased referrals for appropriate speciality care.55 Female subjects are overrepresented among those living in poverty and consequently are disproportionately affected by the disparities in the distribution of wealth, income and access to health-related resources.56 Thus, the underrepresentation of female participants in cardiovascular studies may be partly explained by the lack of access to healthcare and social determinants of health.57,58
Barriers to Remote Patient Monitoring for African-Americans
Technical Factors
Technical considerations may occasionally affect the enrolment of minorities in clinical trials. For example, in the CHAMPION and GUIDE-HF trials, patients were required to have a BMI <35 kg/m2.19,59 Data from the National Center for Health Statistics have demonstrated higher rates of obesity for non-Hispanic African-American patients and women in the US between 2017 and 2018.60 Recent data underscore the importance of central obesity over BMI alone in adversely impacting cardiac function, especially in women.61 Moreover, due to limitations of photoplethysmographic green light signalling, PRM devices using such technology may be accessible only to people with lighter skin tones.62 Green light lacks precision and accuracy and may not read when measuring heart rate in darker skin types.63 This becomes even more important as large-scale trials using commercial wearables become available.45 The results of these studies may not generalise to people of colour and may introduce further bias in the interpretation of the results.
Historical and Societal Factors
The low participation of African-American patients in clinical trials may be influenced by underlying mistrust of the healthcare system. African-Americans have the ongoing experience of racism and segregation, which has contributed to the development of behavioural patterns and beliefs that may keep African-American patients from feeling comfortable in accessing needed diagnostic and therapeutic medical care and in clinical research studies.64 Many black Americans cite the infamous Tuskegee Syphilis Study, the obtainment of Henrietta Lacks’ cervical cells without consent, and James Marion Sims’ experiments as a few examples contributing to their medical mistrust.64 A common perception among African-American patients is that they will be used for experiments in clinical trials. The underlying mistrust is seen not only in research but also in the limited dissemination of RPM.
Furthermore, patient–provider concordance has been shown to enhance adherence to and trust in medical recommendations, particularly among African-American men.65 However, racial diversity is often lacking in the healthcare workforce (Figure 1).66 The lack of diversity may be attributed to various factors resulting from structural racism, such as low-quality secondary education, limited financial support, lack of mentorship and role models, and unreceptive educational environments.67 In addition, patients of colour may be more often perceived as non-adherent, and physicians are less likely to discuss new therapies and clinical trials, indicating a residual bias.68 Adherence is the common endpoint of a complex and multifaceted issue that includes the inability to afford medication, fear of adverse effects, misunderstanding of the need for drugs and an inability to attend medical appointments due to work duties. Consequently, and possibly due to residual implicit bias, there is systemic under-enrolment of African-American patients in clinical trials.69
Socioeconomic Factors
Economic and racial segregation has resulted in lower socioeconomic status among racial and ethnic minority patients, including poor access to and quality of healthcare. These inequalities are likely to contribute to disparities in access to remote monitoring.70 Lower socioeconomic status is associated with an increased incidence of cardiovascular disease even after adjusting for traditional cardiovascular risk factors such as hypertension, diabetes and obesity.71 Lack of exercise facilities, healthy food outlets and institutional resources such as healthcare facilities may explain some of the increased risks for cardiovascular disease and HF seen in people living in deprived neighbourhoods. However, there appears to be a residual risk even when many of these factors are controlled for.72 Multiple socially determined vulnerabilities, including low educational attainment, low annual household income, ZIP code poverty, poor public health infrastructure and lack of health insurance, tend to cluster in the same individuals and increase future HF hospitalisations.73 These social determinants of health account for poor access to organised healthcare structures and new and innovative therapies.
In this context, remote monitoring may be at the forefront of mitigating disparities related to socioeconomic status by providing healthcare access in populations and areas with limited resources. However, this goal may prove challenging due to the lack of appropriate infrastructure in segregated or rural areas and the need for a multifaceted approach in tackling these closely intertwined factors.
How to Improve Equitable Access to Remote Patient Monitoring
Tailored RPM Devices
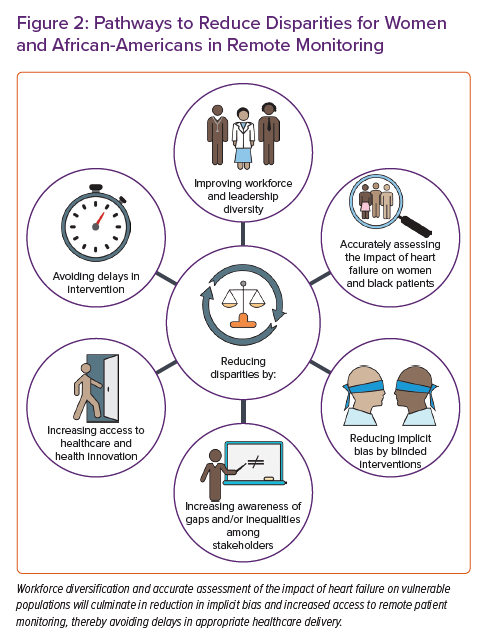
Addressing these previously described factors is paramount (Figure 2). Careful designing of these devices in direct consultation with female leaders on industry steering committees, female patient advocacy groups, tech companies and physicians may be warranted. This process should be designed to address the specific monitoring accuracy, needs and comfort of female participants. Sex-specific design projects have been implemented in different fields of remote monitoring, such as antepartum and perinatal care.74 With regard to cardiac monitoring, radiolucent bra-like garments have been used during exercise stress testing.75 These designs could culminate in the use of bra-like multisensor monitors specifically designed for women.
Eliminating Distrust
In addition, specific disparities for both female patients and African-American patients may pertain to underlying distrust of the medical system. Improving diversity among healthcare staff is crucial in eliminating distrust. Evidence shows that diversification of the healthcare workforce may lead to improved patient outcomes and increased hospital revenue.76,77 The diversification process must disseminate through all levels and layers of healthcare professionals, including physicians, nursing staff, scientists and research coordinators.78 For example, the Faculty Institutional Recruitment for Sustainable Transformation initiative through the NIH aspires to enhance diversity and inclusion among the biomedical faculty.79 The process of workforce diversification is lengthy and would require the contribution of many key stakeholders, including medical schools, nursing colleges, technical schools, hospital administrations and healthcare professionals themselves. Training aiming at bias and racism reduction has increased diversity among healthcare professionals and may improve clinical care delivery.80,81
Policy Changes to Address the Social Determinants of Health
The social determinants of health consist of multiple dynamic components, including insurance coverage, health literacy, education, housing and wealth. To that extent, structural-level and multilevel interventions guided by city- or state-wide policies are more likely to achieve equity and eliminate disparities than individual-level interventions.82 Ensuring policy support for funding of the community health workers is critical to the sustainability of culturally appropriate interventions. Although access to insurance is necessary for providing primary healthcare, it may be insufficient in improving cardiovascular health and eliminating disparities for RPM.83 Ostensible market and health policy changes, as well as payment models focused on longer-term episodes of care and population management strategies, may serve as an impetus for the expansion of RPM in selected patients. This is particularly true if the efficacy of the RPM is around reduction in acute usage (HF hospitalisations, emergency department visits etc.).39
A data-proven clinical benefit of RPM in underrepresented populations would further incentivise commercial and public payers to cover the cost of wearable devices and RPM.84 However, data are lacking, and further efforts to include these newer devices in clinical trials are needed. A few digital health companies are targeting low-income and Medicaid populations as potential markets for expanding their reach.85 Innovative platforms providing wrap-around, community-based services for dual eligible and Medicaid populations may be rapid adopters of efficacious RPM and provide a bridge for existing gaps in RPM coverage.86 A better distribution of medical services in underserved neighbourhoods is also required in addition to insurance access.87 To improve vulnerable patients’ awareness of available medical services, community clinic engagement has been recognised as an effective means of increasing involvement and understanding of underrepresented patients.88
Disparity Reduction Through Technological Innovation and Patient Engagement
RPM is centrally positioned to reduce sex and racial disparities in multiple ways. First, it can theoretically mitigate implicit sex and racial bias through a protocol-driven clinic intervention and the absence of direct patient–clinician interactions, to result in a form of community single blinding outside of the clinical trials context. For example, providers are prompted to treat data directly, reducing potential bias sources. This, in turn, may lead to earlier identification and treatment of subclinical conditions that affect female patients and African-American patients.89,90 Second, RPM may improve healthcare access in underserved areas with poor healthcare structures, eliminating isolation and perceived borders in segregated neighbourhoods. However, the latter may prove challenging given the concurrent absence of other vital infrastructure needed for RPM, such as access to broadband internet. Third, automated patient communication between visits may improve their health engagement and guide them in adhering to their medical care plan. Voice prompts to measure one’s weight or remind one to take their prescribed medication may encourage patients that their condition is professionally managed and reinforce the need to follow essential steps to maintain their well-being. Finally, the delivery of interactive digital material for patient education on their condition could improve outcomes and intensify guideline-directed medical therapies through a sense of patient engagement and self-empowerment in underserved populations.91
Conclusion
As in other cardiovascular diagnostics and therapeutics fields, disparities in the representation and access of women and African-American patients to RPM for the management of HF persist. The research enterprise’s complex technical, socioeconomic, cultural, educational and systemic factors contribute to this phenomenon. The engagement of key stakeholders (female and African-American associations, policymakers) will create the premises for policy transformation targeting equitable access in RPM. Diversification of medical workforce and leadership through a multifaceted involvement (medical and nursing schools, hospital administrations etc.) may improve cultural diversity and improve the inclusion milieu. Adopting alternative pathways of funding with a specific aim to bridge existing gaps will require the demonstration of objective clinical benefit through clinical trials in these populations. Overall, RPM has the unique potential to eliminate disparities through a combination of bias elimination and health equity propagation.