Congestive heart failure (CHF) is a progressive cardiovascular disease with significant morbidity and mortality that affects an increasing amount of people worldwide. There are approximately 6.5 million people in the US, more than 14 million people in Europe, and 26 million people worldwide who are living with heart failure, and the prevalence continues to grow.1–3 In the US alone, there were 960,000 new cases of CHF diagnosed in 2017, and this is expected to continue to increase year on year in the ageing population. It has been estimated that by 2030, the prevalence in the US will exceed 8 million people.4
Along with the high disease prevalence, there is also a significant cost burden related to CHF. The annual worldwide cost of heart failure has been estimated to be US$108 billion, which is about 1–2% of the global healthcare budget.5 The US is responsible for about 28% of the global expenditure, while Europe accounts for about 7%.5,6 In an evaluation of US costs published in 2014, the direct and indirect costs of heart failure were calculated from publicly available resources to be about US$60.2 billion and US$115.4 billion, respectively, significantly higher than previous estimates.7 Given the significant disease prevalence and cost burden, it is essential that healthcare providers investigate multiple therapies to improve clinical outcomes for people with CHF.
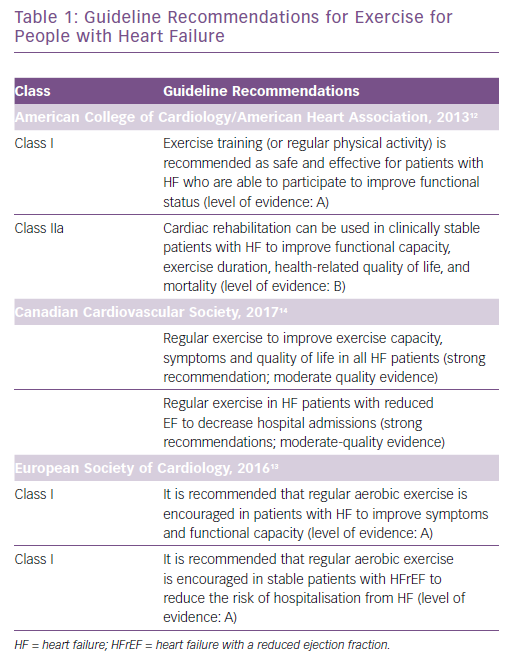
Despite there being many evidence-based therapies that are endorsed by guidelines and have shown to reduce mortality rates and hospitalisations and improve quality of life (QoL) and symptoms, many patients with CHF remain dyspnoeic and fatigued with recurrent hospitalisations, a diminished exercise tolerance and a poor QoL.8 Many studies have shown numerous benefits of cardiac rehabilitation (CR) and exercise training in patients with heart failure, including a reduction in morbidity and mortality.9–11 Guidelines from the American College of Cardiology/American Heart Association, European Society of Cardiology and Canadian Cardiovascular Society have included evidence-based recommendations for the use of exercise in the management of CHF (Table 1).12–14 Additionally, given the data supporting the use of exercise in heart failure as well as the revised guidelines, the US Centers for Medicare & Medicaid Services (CMS) extended coverage for CR for patients with heart failure with a reduced ejection fraction (HFrEF) in 2014.15 Despite inclusion in guidelines and CMS coverage and numerous studies showing clinical benefit from exercise therapy and its safety, it has been underused by people with CHF. It is essential that healthcare providers understand the available literature regarding the safety and clinical benefits related to exercise in this population, as well as the barriers to participation and adherence to CR. It is important that patients are referred to CR programmes and they are encouraged to participate.
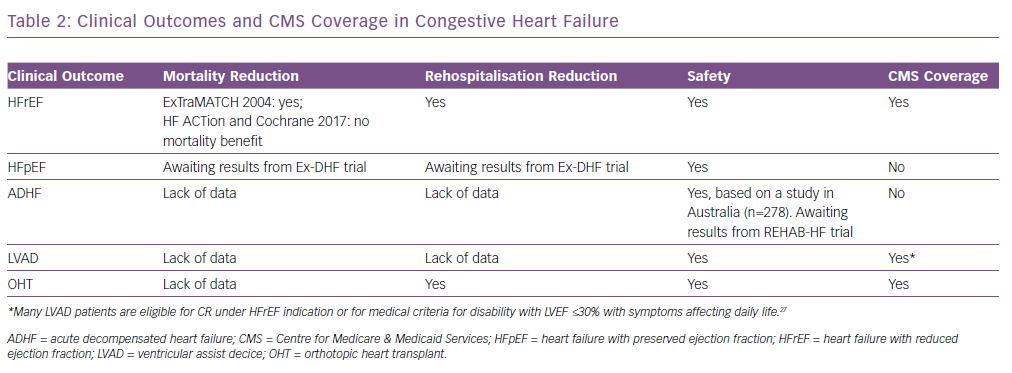
Safety of exercise has been consistently demonstrated in patients with numerous types of clinical HF (Table 2). The Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) trial, which was the largest trial of exercise training in patients with HF with a reduced ejection fraction (HFrEF), investigated the efficacy and safety of exercise for these patients. This was a multicentre, randomised controlled trial that included 2,331 medically stable patients with HF with left ventricular ejection fraction (LVEF) ≤35% and New York Heart Association (NYHA) Class II–VI symptoms despite optimal medical therapy for 6 weeks. Exercise training was demonstrated to be well tolerated and safe for these patients.10 A meta-analysis of 33 trials (including HF-ACTION), involving 4,740 patients with HFrEF with an LVEF <40% and NYHA Class II or III, demonstrated no significant adverse effects of exercise in patients with HF.16 In an evaluation of outcomes for HF with preserved ejection fraction (HFpEF), a meta-analysis that included 276 patients with well-compensated heart failure in six randomised controlled trials demonstrated no major adverse effects of exercise training.17 A study of rehabilitation with 27 patients and another including 278 patients both demonstrated that exercise was safe for patients with acute decompensated HF (ADHF).18,19 The Rehabilitation ventricular assist device (Rehab-VAD) trial and the 2017 Cochrane review of exercise-based cardiac rehabilitation in heart transplant recipients demonstrated the safety of exercising with a LV assist device (LVAD) and orthotropic heart transplant (OHT), respectively.20,21
There has been investigation into the pathophysiology of exercise intolerance in patients with HF and the beneficial effects of exercise training. Mechanisms that may lead to decreased exercise capacity in this patient population include cardiac dysfunction, abnormalities in peripheral flow, endothelial dysfunction, skeletal muscle dysfunction, ventilatory deficits and abnormalities of autonomic nervous system function.22 Exercise capacity is best quantified by peak oxygen consumption (peak VO2) and many studies have demonstrated improvements in peak VO2 with exercise training.9,11,22–24 Additionally, exercise with moderate aerobic training has led to favourable effects on central haemodynamic function, sympathetic tone, peripheral vascular and skeletal muscle function, ventilatory efficiency with decreased dyspnoea and improved QoL.22,25,26
Heart Failure with Reduced Ejection Fraction
The majority of studies investigating the effects of exercise on HF have been related to chronic HFrEF and have demonstrated beneficial clinical outcomes (Table 2). Exercise Training Meta-Analysis of Trials in Patients with Chronic Heart Failure (ExTraMATCH) was a 2004 meta-analysis of nine prospective randomised controlled trials comparing exercise training and usual care in patients with CHF related to LV dysfunction. Significant reductions in mortality and hospitalisations were demonstrated.27 Subsequent systematic reviews have also demonstrated a decrease in hospitalisations, but failed to show significant reductions in mortality.8,28
An updated Cochrane review in 2017, which examined 33 randomised controlled trials including 4,740 participants, predominantly with HFrEF and NYHA Class II and III, demonstrated a reduction in all-cause hospital admissions and HF-specific admissions in up to 12 months of follow-up. Additionally, there was an improved health-related QoL in the exercise training programme group compared with the control.16 There is also evidence to support cost–effectiveness of exercise-based rehabilitation based on two trials included in the review that was attributed to a reduction in hospital bed days.16 The HF-ACTION trial was included in this Cochrane review; it demonstrated safety and an improved QoL among CHF patients randomised to the exercise therapy group.10 Although there was a non-significant reduction in the risk of all-cause mortality and all-cause hospitalisation in this group of patients with chronic HFrEF, there was a risk reduction in the primary endpoint of death or hospitalisation of any cause when adjusted for highly prognostic predictors, including duration of the cardiopulmonary exercise test, LVEF, Beck Depression Inventory II score and a history of atrial fibrillation or flutter. Further sub-study analysis demonstrated that the volume of exercise was a logarithmic predictor of the primary outcome of all-cause mortality or hospitalisation and that there was significant benefit demonstrated from moderate exercise.29
Heart Failure with Preserved Ejection Fraction
Multiple studies have demonstrated safety and effectiveness of exercise for people with HFrEF to improve symptoms, aerobic capacity/endurance and QoL, although people with HFpEF have been under-represented in the studies. Given that HFpEF leads to about 50% of hospital admission for HF and that there is a lack of demonstrated benefit from pharmacotherapies in this patient population, investigation of other potential beneficial interventions for people with HFpEF is essential.16,17 In addition to demonstrating the safety of exercise with no major adverse effects reported in the 276 patients with well-compensated HFpEF in a meta-analysis that included six randomised controlled trials, it was suggested that exercise training improved cardiorespiratory fitness by an increase in peak VO2 and QoL.17 These improvements were noted to be unrelated to a significant change in the diastolic LV function.
The Exercise Training in Diastolic Heart Failure (Ex-DHF) pilot study was a randomised study involving 64 patients that compared supervised exercise or usual care and it demonstrated improvements in exercise capacity and health-related QoL.30 There have been no studies evaluating the effect of exercise on hospitalisations or mortality in the HFpEF population, and HFpEF was excluded from CMS coverage for CR in the most recent decision memo in 2014.15 The Ex-DHF trial, which is currently enrolling participants, is the first multicentre trial to evaluate the long-term effects of exercise on a composite outcome of all-cause mortality, hospitalisations, NYHA functional class, global self-rated heath, maximal exercise capacity, and diastolic function in HFpEF patients.31
Acute Decompensated Heart Failure
There is extremely limited data on the safety and clinical outcomes related to exercise therapy in people with ADHF, which is a leading cause of hospitalisation and is associated with significant morbidity, mortality, and healthcare costs, especially in older patients. These patients have been excluded from previous exercise training trials and the updated CMS memo for CR coverage from 2014.15
The Rehabilitation Therapy in Older Acute Heart Failure Patients (REHAB-HF) pilot study provided feasibility of an ongoing multicentre, randomised, attention-controlled trial funded by the National Institute of Health to evaluate the use of rehabilitation to improve physical function and reduce rehospitalisations for patients ≥60 years beginning in the hospital during an admission for ADHF (including HFrEF and HFpEF) and continuing for 12 weeks after discharge.18 This pilot study included 27 patients with admissions for ADHF that were randomised into a novel rehabilitation intervention group, focusing on improved balance, strength, mobility and endurance, an attention control group or usual care, and demonstrated feasibility, safety and a trend toward improved physical function and decreased hospitalisations in the intervention group. Given that this is a pilot study with a small sample size of the larger and randomised controlled trial (REHAB-HF) that is currently enrolling participants, the authors recommend caution in instituting immediate rehabilitation in older patients with ADHF.
The Exercise Joins Education: Combined Therapy to Improve Outcomes in Newly-discharged Heart Failure (EJECTION-HF) trial was a multicentre randomised controlled trial in Australia that included 278 recently discharged CHF patients who were randomised to 24 weeks of supervised centre-based exercise therapy commencing within 6 weeks of discharge or standard care.19 Average time to initiation of CR in these patients was 43 days and there were no adverse events associated with the therapy, suggesting that exercise therapy in patients recently hospitalised with acute HF is safe and feasible. Adherence in the home exercise group was 75% at 3 months and 68% at 6 months, while the centre-based exercise group had poor adherence with only 43% of patients participating in ≥50% of the sessions. There was no difference in the primary outcome of all-cause death or readmissions, although there was a significant reduction in all-cause mortality in the exercise group (based on a small number of events), which should be interpreted with caution. The results of the REHAB-HF trial will provide additional insight into the benefit of early rehabilitation for patients with ADHF.
Left Ventricular Assist Devices
Patients that have been implanted with LVADs are reported to have improved survival, functional capacity and health status, although many continue to report exercise intolerance and heart failure symptoms. The Rehab-VAD trial is the largest prospective randomised trial of the beneficial effects of exercise on LVAD patients. It included 26 patients randomised to CR or usual care after implantation of an LVAD. It demonstrated that exercise was safe in the CR group with only one event (syncope) in more than 300 sessions, and showed an improved total treadmill time, muscle strength and improved health status (evaluated by the Kansas City Cardiomyopathy Questionnaire) with continuous flow LVADs compared with usual care.20 There was no difference in the peak VO2, which has been a marker of exercise capacity in people with CHF, although additional studies have suggested an improvement in VO2 with exercise therapy after VAD implantation.32 A recent study of 1,164 Medicare beneficiaries receiving LVADs demonstrated low participation in CR (30%). Of those who participated in CR, there was a decreased risk of hospitalisation and mortality at 1 year after multivariate adjustment with a 23% and 47% reduction, respectively, compared with those who did not participate in CR.33 This was not the primary outcome of this study and there were likely additional confounding variables, although it suggests potential clinical benefits and identifies a need for further studies to evaluate the value of exercise in people with LVADs (Figure 1A).
Cardiac Transplantation
Although there have been significant improvements in OHTs over the past 40 years, long-term survival remains limited. Exercise capacity and health-related QoL in transplant recipients have been noted to be inferior compared with age-matched healthy people.34 In the past, transplant patients were advised not to exercise due to concerns of chronotropic incompetence in the denervated heart, although further studies have shown evidence of sympathetic reinnervation, which is associated with improved exercise capacity and may be improved by physical training.35 An updated Cochrane review in 2017 included ten randomised controlled trials with 300 patients who had OHTs demonstrated the safety of exercise therapy in transplant patients with only one reported adverse event. Nine studies compared exercising to control and one study compared high-intensity to moderate-intensity training.21 CR participation was associated with an improvement in peak VO2 and exercise capacity, although there was no significant improvement in health-related QoL in a 12-week period. There was no data to report hospitalisations or mortality benefit in these studies. Additional studies have demonstrated improvement of peak heart rate, ventilatory capacity, autonomic function and QoL with exercise training.36 In an evaluation of CR and readmission rates for 595 Medicare beneficiaries that received heart transplants in the US in 2013, 55% of patients were enrolled in CR. Participation in CR was associated with a 29% lower readmission risk at 1 year.36 Younger patients (aged 35–49 years) were significantly less likely to enrol in CR, and those that enrolled were likely to attend fewer sessions that patients older then 65 years. There have been no published studies investigating the effects on mortality of OHT patients who have participated in exercise training or CR. Given the significant benefits of CR and the CMS coverage of CR in orthotopic heart transplant patients that was approved in 2006, there should be a significant effort to improve uptake of CR in this patient population (Figure 1B).37
CMS Coverage
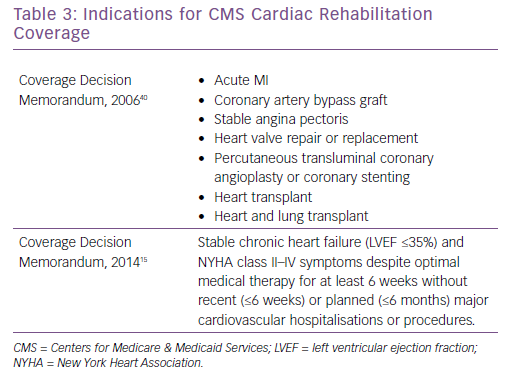
In 2006, CMS published a decision that there was adequate evidence to approve coverage of CR for patients with an acute MI, coronary artery bypass graft, stable angina, heart valve repair or replacement, percutaneous transluminal coronary angioplasty or coronary stenting, and heart or heart and lung transplant (Table 3).37 At that time, there was insufficient evidence to approve CR coverage for CHF patients. After numerous studies were published demonstrating benefit of exercise training for patients with HFrEF, the largest of which being HF-ACTION, CMS expanded coverage for stable, chronic HF defined as patient with an LVEF ≤35% with NYHA II–IV symptoms despite optimal medical therapy for at least 6 weeks without recent or planned hospitalisation or procedure.15 Specific CR coverage is not available for patients with HFpEF, ADHF or LVAD, although many LVAD patients are eligible for CR under the HFrEF indication, or by medical criteria for disability with LVEF ≤30% with symptoms affecting daily living.33 Although HFpEF patients represent a significant number of CHF patients and hospital admissions, and ADHF is a significant cause of morbidity, mortality and is a component of healthcare expenditures, there is currently no CMS coverage for CR for these patients. Additional studies, including the Ex-DHF trial for HFpEF and REHAB-HF trial for ADHF, are necessary to demonstrate safety and clinical benefit to encourage CMS coverage for CR.18,30,31
Uptake and Adherence
Despite numerous benefits and CMS coverage for many patients, there has been significant underuse of CR for people with CHF. An earlier study demonstrated that only 10.4% (12.2% HFrEF, 8.8% HFpEF) of 105,619 eligible patients with HF (48% with HFrEF, 52% with HFpEF) received a CR referral after hospitalisation for CHF.38 In the HF-ACTION trial with HFrEF patients, despite numerous methods to reinforce adherence, about 30% of those enrolled in the exercise arm exercised at or above the target goal.10 A retrospective study using the CMS and the Veterans Health Administration (VA) national data between 2007 and 2011 evaluated CHF patient enrolment in one or more sessions of CR. Of the 66,710 veterans and 243,208 Medicare beneficiaries hospitalised for HF, 2.3% and 2.6% respectively, attended one or more sessions of outpatient CR.39 The investigators noted that they were unable to determine the prevalence of HFrEF that would be eligible for CR in these populations by using the ICD-9 codes. Much of the US data was collected before CMS coverage expansion of HFrEF in 2014. For LVAD and OHT recipients with Medicare coverage, uptake of CR was 30% (of 1,164 LVAD patients) and 55% (of 595 OHT patients).33,36 In a 2010 European survey, it was reported that <20% of HF patients were participating in CR.40
There are many potential barriers involving either the healthcare system or patient adherence that influence the use of CR. The healthcare provider should understand that current guidelines, consensus statements and high-impact studies demonstrate the value of exercise training, in addition to confirming available CR sites with educated CR teams. Additionally, many patient factors, including socioeconomic factors, work conflicts, inadequate transportation, lack of reimbursement, significant symptoms, as well as patient attitude, beliefs and motivations, affect enrolment and adherence to CR.43 In many cases, there are multiple barriers that need to be addressed to significantly improve CR use in people with CHF.
Conclusion
CHF is an increasingly prevalent disease with significant morbidity and mortality despite optimal drug and device therapies. Exercise training and cardiac rehabilitation have demonstrated numerous benefits for people with CHF, including improved exercise capacity and QoL, in addition to improved clinical outcomes. Exercise has also been established as safe and feasible with HF and, in some studies, exercise therapy has demonstrated improved cost-efficiency in HF management. The majority of current studies and subsequent guidelines have been established based on the benefits of exercise in HFrEF patients, although further studies are necessary to evaluate clinical outcomes with exercise in different HF populations to drive expansion of the guidelines to include HFpEF, VAD and OHT patients. Despite numerous benefits in multiple HF groups, there is significant underuse of CR due to many barriers that need to be overcome. Healthcare providers should strongly consider referring their patients with CHF to CR and encouraging participation in and adherence to exercise training programmes.