Cardiac allograft vasculopathy (CAV), a potential complication after cardiac transplantation, presents as a diffuse, progressive thickening of the myocardial arteries and remains a major cause of increased morbidity and mortality after transplant due to the development of ventricular dysfunction and life-threatening arrhythmias.1 The prevalence of CAV increases with increased duration of graft survival, with rates of 8%, 29% and 47% at 1, 5 and 10 years following cardiac transplantation.2 Invasive techniques, such as coronary angiography and IV ultrasound, are gold standards for diagnosis of CAV, although the use of non-invasive imaging such as stress echocardiogram and myocardial perfusion imaging is on the rise.
Both immunological and non-immunological factors have been associated with an increased risk of CAV. Immunological risk factors include differences in donor and recipient human leukocyte antigen (HLA), presence of alloreactive antibodies and episodes of acute rejection.2 T-cell activation leads to expression of adhesion molecules on the surface of endothelial tissues.2 Non-immunological factors, such as hyperlipidaemia, hyperglycaemia and history of cytomegalovirus viraemia or infection, have all been determined to be independent risk factors for the development of CAV.3 Various medication therapies are used in modern clinical practice to reduce CAV risk or delay its progression including aspirin, mammalian target of rapamycin (mTOR) inhibitors and 3-hydroxy-3methylglutaryl coenzyme-A (HMG-CoA) reductase inhibitors (statins).
A prospective, randomised open-label trial of 97 heart transplant recipients showed that the use of pravastatin 40 mg daily after cardiac transplant led to a reduction in cholesterol levels, a lower incidence of CAV, and increased patient survival.4 A 10-year follow-up to this study demonstrated similar effects, with increased 10-year graft survival and 10-year freedom from CAV and death.5 The beneficial effects of statins were verified with a randomised controlled trial of simvastatin, up-titrated to a dose of 20 mg per day, compared with diet alone, which demonstrated a significant reduction in LDL, lower incidence of CAV and improved 4-year patient survival.6 A recent retrospective analysis demonstrated lower change in plaque index and decreased risk of CAV-associated events with early initiation of statins (defined as less than 2 years after transplant) compared with late initiation in the context of modern immunosuppression and diagnostic techniques.7 The cardiovascular benefit associated with statins has been hypothesised to be due to their effect on lowering total cholesterol and LDL. A 2018 retrospective cohort analysis evaluated the relative risk of developing CAV with respect to LDL reduction and found that patients who achieved a median LDL of <2.6 mmol/l had a delay in time to CAV. This benefit was not seen with an LDL goal of <1.8 mmol/l.8
In the non-transplant population, the focus on prevention of atherosclerotic cardiovascular disease has shifted from targeting specific LDL goals to placing patients on higher intensity statins.9,10 In the transplant population, the use of specific statin medications and doses can be limited due to pharmacological interactions with calcineurin inhibitors (cyclosporine and tacrolimus) and other post-transplant medications. These drug interactions can increase statin exposure and place patients at risk for myopathy and rhabdomyolysis.11–14 Pharmacokinetic studies have evaluated the interaction of cyclosporine with high-intensity doses of atorvastatin and rosuvastatin, and noted a 6–15-fold and 7.1-fold increase in the atorvastatin and rosuvastatin areas under the curve, respectively.15,16 Unlike cyclosporine, tacrolimus is only a substrate and not an inhibitor of cytochrome P450 3A4, and is therefore theoretically safer in combination with higher intensity statins. A retrospective study of 24 heart transplant recipients receiving tacrolimus therapy showed that high-intensity statins were well tolerated, with only one patient experiencing myalgias and none experiencing rhabdomyolysis or hepatotoxicity.17
A retrospective study of 346 patients found that greater statin intensity significantly prolonged time to a composite primary endpoint of heart failure hospitalisation, revascularisation, MI or death.18 Another report of 131 heart transplant patients found no association between high-intensity statins and the incidence of CAV at 1 or 3 years.19 Although statin intensity has been associated with reduction in atherosclerotic cardiovascular disease in non-transplant patients, the effect of statin intensity on CAV reduction in the cardiac transplant population is still unknown. The primary aim of this study is to evaluate the effect of statin intensity on the time to the development of CAV after cardiac transplantation.
Methods
Design and Clinical Protocol
This single-centre retrospective cohort analysis was approved by the Vanderbilt University Medical Center Institutional Review Board. Analyses were conducted in late 2018 and early 2019. Adults (age ≥18 years) were included if they: received an orthotopic heart transplant at our institution between February 2013 and April 2017, thus allowing for at least 12 months of potential follow-up; were managed after transplant at our institution; began statin therapy within 1 year after transplantation; and had at least one cardiac angiogram and one lipid panel after transplantation. Multi-organ transplant recipients, those with a history of previous heart transplant, and recipients of hepatitis C-positive organs were excluded.
Based on our institutional protocol, all heart transplant recipients are placed on tacrolimus with a tacrolimus trough goal of 8–12 ng/ml, mycophenolate mofetil 1,000 mg every 12 hours, and prednisone taper with therapeutic alterations based on the patient’s individual post-transplant course. Unless contraindicated, patients are also started on statin therapy prior to discharge from their transplant hospital admission. The choice of statin agent is based on the patient’s statin therapy prior to transplant and on their baseline lipid panel. Per protocol, patients who had non-ischemic cardiomyopathy as the indication for transplant had a post-transplant LDL goal of < 2.6 mmol/l, and patients with a history of ischaemic cardiomyopathy prior to transplant had an LDL goal of <1.8 mmol/l. Doses are increased until patients achieve their LDL goal, or they are intolerant to therapy. Lipid panels are evaluated every 3 months for those who remain above their LDL goal or who have any change in statin therapy. Coronary angiography is obtained at 1, 3 and 5 years after transplantation unless contraindicated by severe renal impairment, defined as an estimated glomerular filtration rate <30 ml/min/1.73m2. Coronary angiography may be obtained earlier, and more frequently, if there is a clinical suspicion of CAV.
Data Encoding
Statin therapies were classified as low, moderate and high intensity, based on American College of Cardiology and American Heart Association classifications.20 They were stratified, for the purpose of these analyses, as low and moderate/high due to the small proportion of patients receiving high-intensity statins. The presence or absence of CAV was defined according to the 2010 International Society of Heart and Lung Transplant standardised nomenclature using coronary angiography only, which was reviewed by both interventional and transplant cardiologists.21 The CAV follow-up period was defined as the time (in months) from the transplant to the determinative CAV follow-up date, which was either the date of the first or only CAV-positive coronary angiography (CAV-1, CAV-2, or CAV-3), or the date of the last angiography that was CAV negative (CAV-0).
Demographic and clinical data collected included age, history of diabetes, history of hypertension, history of chronic kidney disease, indication for transplant, donor and recipient cytomegalovirus serology, and smoking history. Lipid panels and HbA1c data were collected at baseline, which was defined as 2 weeks prior to/after transplantation, and longitudinally after transplant. Dyslipidaemia therapy was assessed immediately prior to transplantation, at discharge, and annually. Statin intensity was determined based on the medication and dose that was closest to and ≤6 months before or after the CAV follow-up date. Immunosuppression was documented at discharge and annually after transplantation. Non-CAV outcomes, such as post-transplant lipids, were monitored throughout the CAV follow-up period, with a tolerance of 14 days following the CAV follow-up date. The number of biopsy-proven rejection episodes with a grade greater than or equal to 2R in the CAV follow-up period was tallied and coded as a binary variable, the presence or absence of rejection. Within-subject median post-transplant values for lipids and HbA1c were calculated for those patients having at least two data points in their CAV follow-up period.
Statistical Analysis
Differences in demographic and clinical characteristics based on statin use and intensity groups (none, low, moderate/high), and in post-transplant measures between statin intensity groups (low, moderate/high) were evaluated using analysis of variance or χ-squared tests, with z-tests of column proportions. Kaplan–Meier survival methods with the log rank test were used to evaluate CAV-free survival in the entire cohort, between those who were and were not receiving statin therapy, and the effect of statin intensity (low versus moderate/high) in patients receiving statin therapy. Cox proportional hazards regression was used to test the association between within-subject median post-transplant LDL and CAV-free survival. Analysis of co-variance was used to test the differences between median post-transplant total cholesterol, LDL, HDL, triglycerides and HbA1c between those with and without CAV, and between the three statin groups (none, low, moderate/high) after adjusting for CAV follow-up time. Multivariable logistic regression was used to test the effect of rejection on the likelihood of CAV after adjusting for CAV follow-up time.
Some study data were collected and managed using Research Electronic Data Capture (REDCap) tools hosted at Vanderbilt University Medical Center. REDCap is a secure, web-based application designed to support data capture for research studies.22 All analyses were conducted using IBM SPSS (version 25.0; IBM) and statistical significance was indicated if a non-directional p-value was less than 0.05.
Results
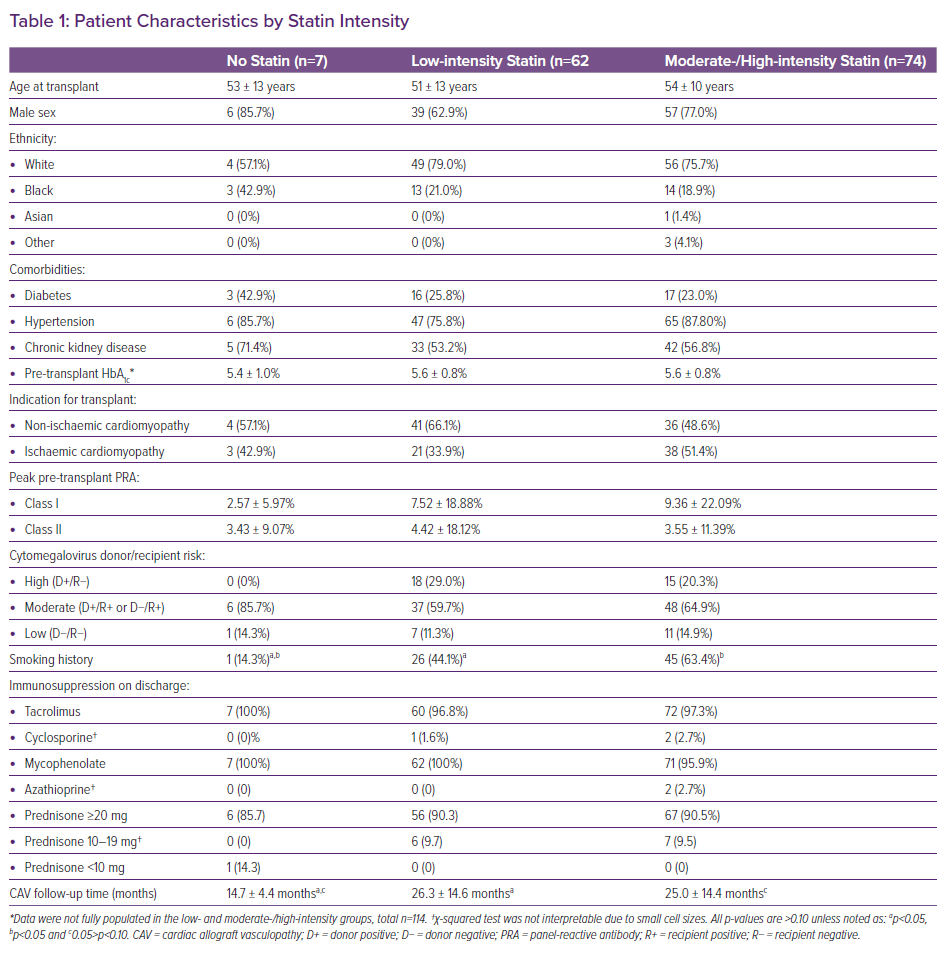
In total, 217 adults underwent transplantation between February 2013 and April 2017. Of those, 74 (34%) were excluded. Sixty-five people (30%) met a single exclusion criterion: multi-organ transplant (n=10, 5%), previous heart transplant (n=5, 2%), followed up at a different institution (n=33, 15%), and unavailable cardiac catheterisation data (n=17, 8%); and nine people (4%) were excluded for two or more reasons. Of the 143 people included in the primary analysis, seven were not on a statin, 62 were on a low-intensity statin and 74 patients were on a moderate- or high-intensity statin at the CAV follow-up date. Agents that were included in this cohort included atorvastatin, pravastatin, simvastatin, rosuvastatin and pitavastatin. As shown in Table 1, with the exception that a higher proportion of patients in the low-intensity and moderate-/high-intensity groups had a smoking history prior to transplantation compared with the no statin group, there were no statistically significant differences in baseline characteristics between the three groups (all p>0.10). Recipients were predominantly white, male, and had an average age of 53 years. The mean panel of reactive antibody for both Class I and Class II was less than 10% in all groups. The majority of patients were discharged after transplantation on tacrolimus, mycophenolate and prednisone.
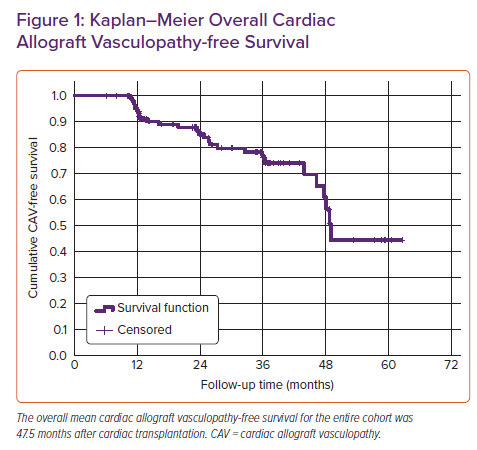
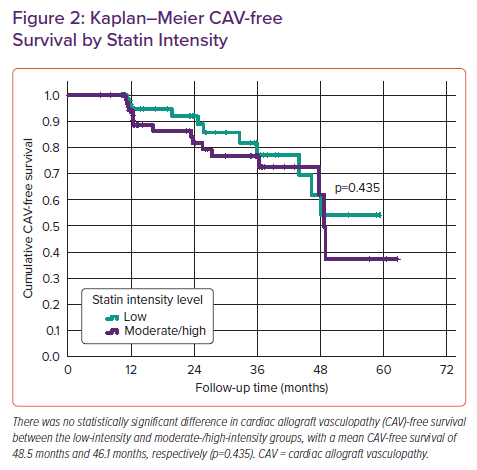
On analyses of post-transplant outcomes, 29 (20%) were diagnosed with CAV-1 or greater and 114 remained CAV free over the total follow-up period, which averaged 25.1 ± 14.4 months (range, 6.1–62.6 months). Mean CAV-free survival was 47.5 months (95% CI [43.1–51.8]) (Figure 1). Although the sample was substantively smaller and the follow-up time substantively shorter in the group that did not receive statin therapy (Table 1), treatment with a statin after transplantation showed a trend towards improved CAV-free survival compared with those who did not receive statin therapy (p=0.055). There was no statistically significant difference in CAV-free survival between the two statin groups, with a mean CAV-free survival of 48.5 and 46.1 months in the low- and moderate-/high-intensity statin groups, respectively (p=0.435) (Figure 2).
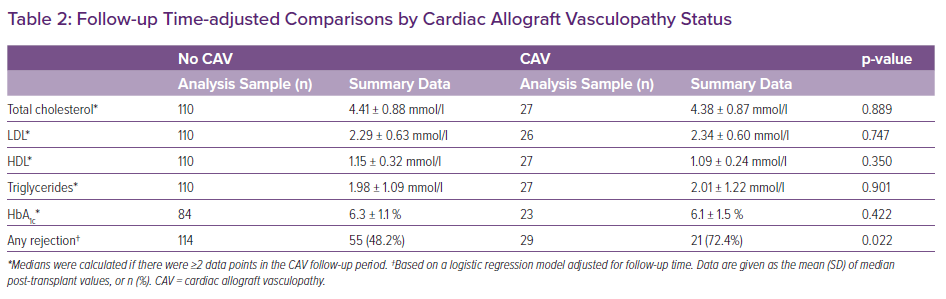
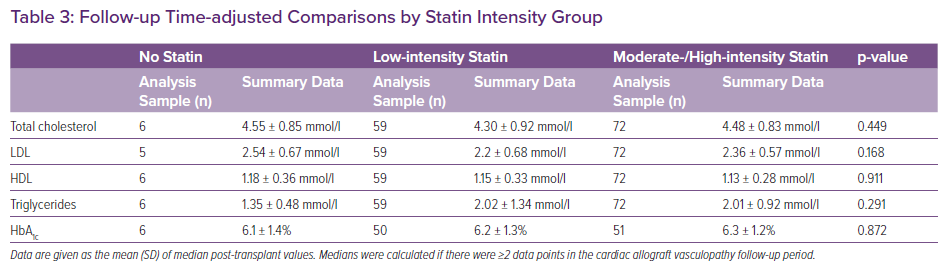
In those for whom it could be calculated (n=136), median post-transplant LDL was not associated with time to CAV (p=0.790). This lack of association was also reflected when median post-transplant LDL was stratified as <1.8, 1.8 to 2.5, and ≥2.6 mmol/l (all log-rank p≥0.467). Related analyses showed that, after adjusting for follow-up time, median post-transplant LDL in those who developed CAV compared with those who were CAV free averaged 2.34 ± 0.6 mmol/l and 2.29 ± 0.63 mmol/l, respectively (p=0.747) (Table 2). Similarly, after adjusting for follow-up time, there were no statistically significant differences in median post-transplant total cholesterol, HDL, triglycerides or HbA1c between those with and without CAV (all p≥0.350). After adjusting for CAV follow-up time, patients who had at least one rejection episode were 2.9-fold more likely to have CAV than those who remained rejection free (HR 2.87; 95% CI [1.17–7.04; p=0.022). Average LDL in the no statin, low-intensity, and moderate-/high-intensity statin groups, after adjusting for follow-up time, was also not statistically significantly different, at 2.54 ± 0.67 mmol/l, 2.20 ± 0.68 mmol/l and 2.36 ± 0.57 mmol/l, respectively (p=0.168; Table 3).
Discussion
Previous studies have demonstrated benefits of statins in prolonging survival and reduction in CAV progression; however, many evaluated them as a class effect, without evaluating the differences in dosing regimens.4–6 This study has demonstrated that, irrespective of the statin intensity, patients had similar CAV-free survival durations, given that there was no difference in time to CAV between the low-intensity and moderate-/high-intensity statin groups. These data add to the limited literature regarding whether statin intensity has an impact on clinical outcomes after heart transplant. Given the drug–drug interactions that exist between calcineurin inhibitors, other post-transplant medications, and statins, patients may equally benefit from lower dose statins to mitigate the risk of drug-related adverse effects. Despite being on different intensity statins, patients in the low-intensity and moderate-/high-intensity groups had statistically similar median post-transplant LDL levels of 2.2 mmol/l and 2.35 mmol/l, respectively. This affects the applicability of placing all patients on low-intensity statins after heart transplantation, and would only be applied to the group of patients who achieve an LDL <2.6 mmol/l on a low-intensity statin.
There was no association between median post-transplant LDL and time to CAV, suggesting that the benefit of statins may be independent of LDL reduction. It has been previously hypothesised that the effects of statins on CAV progression may be impacted by mechanisms independent of their effect on atherosclerosis through reduction in cholesterol deposition.23–25 Non-lipid-related mechanisms have been proposed based on animal models that include attenuation of vascular smooth muscle proliferation, downregulation of growth factor genes in smooth muscle cells, and downregulation of endothelial nitric oxide production.23–26 This conclusion cannot be fully applied to our cohort given that patients in both statin intensity groups had a median LDL level <2.6 mmol/l, which has been previously shown to delay time to CAV.8
The two statin intensity groups were well balanced and the only statistically significant difference in the baseline characteristics was smoking history prior to transplant, which was higher in the statin groups. On univariate analysis to evaluate the risk factors for CAV there were no differences in post-transplant median total cholesterol, LDL, HDL, triglycerides or HbA1c. The only significant effect identified was that patients who had rejection grade 2R or greater were approximately threefold more likely to develop CAV, which aligns with previously published studies.26,27
The limitations of this study include those inherent to single-centre, retrospective designs. Post-transplant monitoring was not uniform for all of the patients; however, all available data between the transplant and the determinative CAV follow-up date were collected, and differences in follow-up time were addressed through survival and co-variance-adjusted statistical methods. The timing and duration of mTOR inhibitors were difficult to capture. However, at our institution, mTOR inhibitors are frequently started in patients who develop CAV and therefore they do not interfere with CAV-free survival in this cohort. Detection of CAV in this cohort was mostly done using cardiac catheterisation and not intravascular ultrasound, therefore, the sensitivity of CAV detection may be reduced in this study. Finally, baseline and donor angiography were not analysed, meaning that the effects of pre-existing disease cannot be determined.
Conclusion
HMG-CoA reductase inhibitors, as a class, have been shown to be beneficial in treating dyslipidaemia and preventing CAV after heart transplantation. Guidelines for the prevention of atherosclerotic cardiovascular disease in non-transplant patients recommend the use of high-intensity statins. Whether high-intensity statins convey a similar benefit in heart transplant recipients is unknown. Our study showed no difference in time to CAV between heart transplant recipients treated with low intensity compared with moderate-/high-intensity statins. Our data suggest that patients may have prolonged CAV-free survival while being on a statin therapy that provides adequate LDL reduction irrespective of statin intensity. A larger, prospective study is needed to confirm these findings.