The term ‘biomarker’ encompasses a wide-ranging subcategory of objective biological information, which can be measured accurately and reproducibly and serve as indicators of normal or pathological processes or responses to therapeutic interventions.1 In the context of acute heart failure (AHF), biomarkers play a crucial role in several aspects of the patient journey, such as facilitating prompt and accurate diagnosis, risk stratification, monitoring and guiding the initiation or titration of appropriate treatment.2 However, for a biomarker to be clinically useful, it should possess several key features:
- high diagnostic accuracy;
- it should reflect a critical pathophysiological pathway involved in the disease process;
- the assays used should be robust and reproducible;
- have a rapid turnaround time to facilitate timely clinical decision-making at the point of care;
- be economically viable; and
- complement clinical judgement in enhancing the understanding of diagnosis, prognosis or management.1
Therefore, despite the field of biomarkers in heart failure (HF) being dynamic, only a few have translated from initial promise/preclinical use to being integrated into European Society of Cardiology or American College of Cardiology/American Heart Association guidelines.3,4
In this article, we will discuss the clinical role of established and emerging circulating biomarkers in the context of AHF, focusing on its practical use in daily clinical care.
Biomarkers for the Diagnosis of Acute Heart Failure
An accurate and timely diagnosis of AHF in the emergency department (ED) is essential to ensure early and appropriate treatment. Some patients may have a more straightforward presentation with easily identifiable symptoms or signs on physical examination and imaging, such as chest X-ray or lung ultrasound, which facilitates an accurate diagnosis. However, others may not fit the classic clinical picture or exhibit symptoms or other coexisting conditions, which leads to diagnostic uncertainty. In such cases, combining clinical judgement with the use of biomarkers may improve diagnostic accuracy, enabling prompt initiation of appropriate management.5
Natriuretic Peptides
Natriuretic peptides (NP) – B-type natriuretic peptide (BNP), N-terminal proBNP (NT-proBNP) and mid-regional pro-atrial natriuretic peptide (MR-proANP) – are by far the most extensively studied and most widely used biomarkers in AHF and current clinical practice guidelines recommend their measurement for early diagnosis or exclusion of AHF, particularly if the diagnosis is uncertain and an assay is available at the point of care.3,4
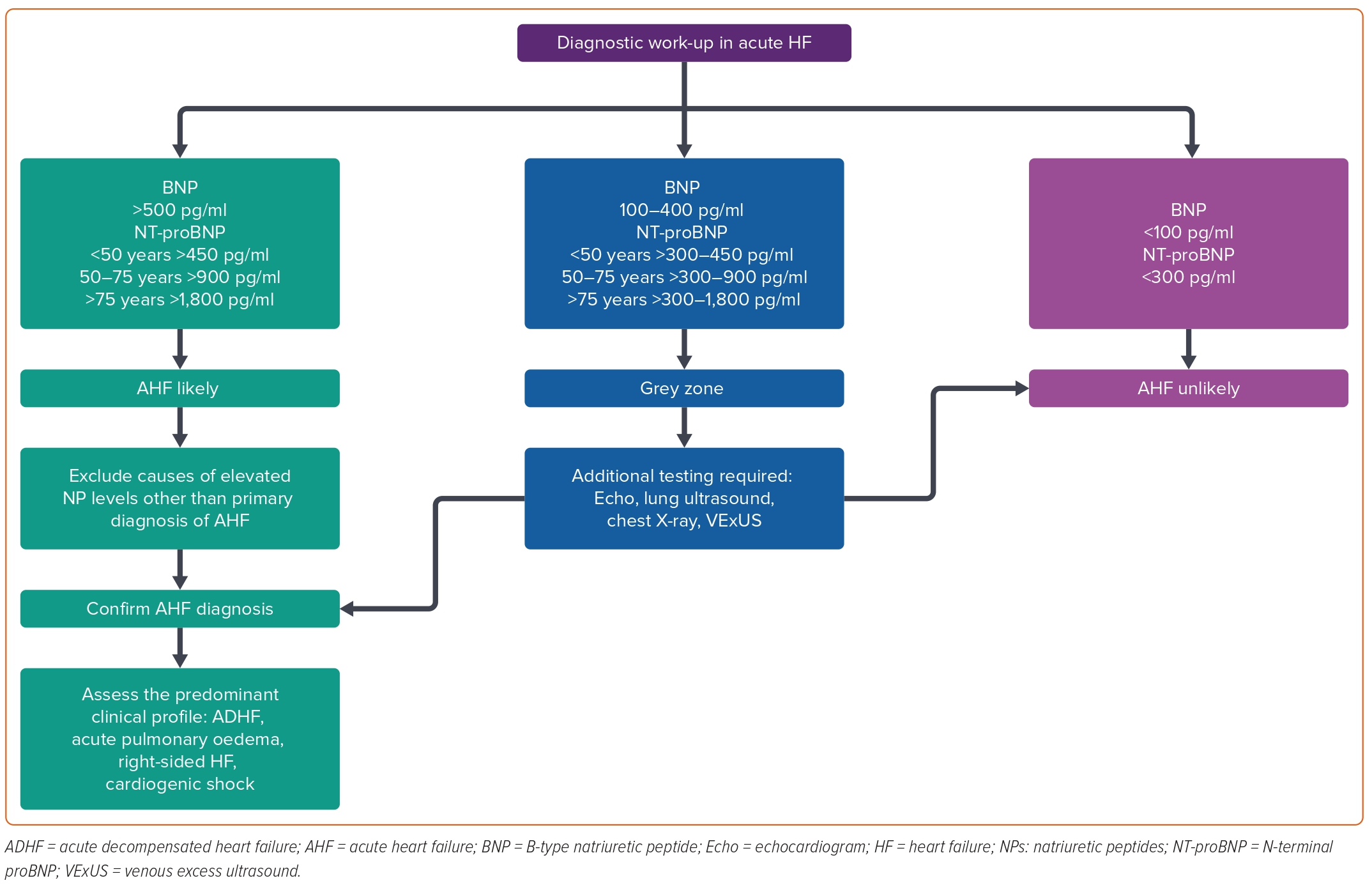
Guidelines suggest specific cut-off values for BNP and NT-proBNP to aid in the initial diagnostic work-up.3,4 In patients with suspected AHF, a cut-off concentration of <100 pg/ml BNP and <300 pg/ml NT-proBNP provide an excellent negative predictive value to exclude the presence of HF.6 Concentrations above the ruling-in cut-off values support the diagnosis of AHF, particularly if clinical signs and symptoms are consistent.6 The age-dependent ruling-in cut-off values for BNP are >400 pg/ml, while the age-dependent values for NT-proBNP are <50 years: 450 pg/ml, 50–75 years: 900 pg/ml, and >75 years: 1,800 pg/ml; Figure 1).6 However, it is important to recognise that these cut-off values are not absolute and the diagnostic performance is maximised when NPs are evaluated as continuous variables, especially in patients with an intermediate pre-test probability. Thus, very low concentrations have a high negative predictive value for excluding the presence of HF. At the other extreme, the higher the NP concentration, the higher the likelihood of an accurate diagnosis of AHF.
However, despite the strong evidence supporting the use of NPs to assess AHF, some considerations should be acknowledged when interpreting BNP or NT-proBNP levels in patients presenting with worsening HF symptoms.
Higher-than-expected Natriuretic Peptide Levels
NPs are not specific to HF and heart disease.7 Therefore, when faced with elevated NP levels in the ED, it is crucial to consider alternative conditions that may lead to increased concentrations of these peptides, particularly in patients with a low pre-test probability or when an alternative diagnosis seems more plausible.8 Such conditions include those that result in increased myocardial wall stress without a direct association with fluid retention, such as AF, acute coronary syndrome, infection, high-output states and pulmonary embolism, and those related to diminished clearance (renal failure).9–11 Nevertheless, it must be emphasised that extreme values should not be overlooked because they may identify the presence of unrecognised or masked HF and it is essential to acknowledge that patients often do not present with a single diagnosis. It is important to contextualise the results according to the patient’s profile and clinical presentation.
Lower-than-expected Natriuretic Peptide Levels
Transmural wall stress is the most potent trigger for NP synthesis and release.12 In the setting of AHF, the primary factor contributing to decompensation is either volume overload and/or fluid redistribution in a heart that cannot adequately accommodate an increase in preload volume, subsequently leading to a pronounced rise in cardiac filling pressures.13 As such, most NP release stimuli in AHF are predominantly driven by volume. Laplace’s law governs wall stress and states that for a particular intracavitary pressure, wall stress is directly proportional to the size or radius of the cavity and inversely proportional to the thickness of the left ventricular (LV) wall. Therefore, in patients with HF with preserved LV ejection fraction (HFpEF), the LV chamber size is typically smaller and thicker, while those with predominantly right-sided HF have a lower ventricular mass. As a result, HFpEF and right-sided HF patients often exhibit lower NP values for the degree of systemic congestion compared with patients with reduced LV ejection fraction (LVEF). Therefore, it is important to consider these factors when interpreting NP levels in patients with different types of HF, as these values may not always reflect the severity of the condition.14
Rule-In/Rule-out Cut-off and Baseline Values
Although both BNP and NT-proBNP have validated rule-out and rule-in cut-off thresholds, the most robust evidence for NPs in AHF lies in their high negative predictive value (rule-out).6 Furthermore, it is common to encounter patients with NP values that fall between established diagnostic cut-offs for rule-out and rule-in – a grey zone that complicates the differentiation between HF and alternative conditions. This scenario is frequently observed in people with obesity who typically exhibit lower NP levels than their non-obese counterparts, potentially leading to false-negative results and a delay in diagnosis and appropriate treatment.15,16 In such scenarios, combining NP measurements with other diagnostic tools, such as echocardiography or lung ultrasound, may help improve diagnostic accuracy.5 Similarly, it is essential to recognise that, when considering rule-in thresholds, certain patients with chronic HF may exhibit persistently elevated NP values as a result of structural or functional abnormalities rather than necessarily indicating an acute haemodynamic change. Familiarity with each patient’s NP concentration under stable conditions – referred to as the ‘dry’ NP concentration – assists in interpreting these biomarker levels when patients present with acute symptoms. A significant increase in NP from dry values (>100%) may indicate a shift in the clinical state, such as worsening HF (Figure 1).7
Ultimately, it is crucial to adopt a comprehensive approach, using NP concentrations as one piece of the diagnostic puzzle. Integrating patient history, clinical presentation and additional diagnostic tools will enable a more accurate diagnosis and better management of the patient’s condition.
Cardiac Troponin
High-sensitivity cardiac troponin (hs-cTn) testing is commonly conducted in patients presenting with AHF syndromes to exclude the presence of type 1 MI. In this scenario, a significant elevation – >10 times the upper limit of normal (ULN) – and/or substantial increases within 1–3 hours >100 ng/l should alert for the possibility of concurrent myocardial ischaemia.3,4 In its absence, cardiac troponin elevation should be regarded as a myocardial injury due to subendocardial stress or myocyte degeneration. Notably, elevated hs-cTn levels — regardless of the presence or absence of coronary artery obstruction — prognosticate the risk for progressive ventricular remodelling and predict an increased mortality risk.17–20
Biomarkers for Monitoring Diuretic Response and Decongestion During Admission
Achieving complete decongestion before hospital discharge is an important therapeutic goal in AHF.29 However, accurately assessing a patient’s response to decongestive therapies and determining the optimal time to discontinue these treatments remains a significant challenge. Although weight loss and clinical scores based on physical examination are frequently used to evaluate the congestion status, both have limited value and often require additional biomarkers and imaging modalities to provide a more comprehensive assessment.
Urinary Sodium
In the setting of congestion with volume overload, measuring a spot urine sodium content 1–2 hours following loop diuretic administration has shown to be an excellent marker to predict the initial diuretic response.29 A spot urine sodium content of <50–70 mEq/l generally identifies a patient at risk of poor diuretic response for whom doubling the initial loop diuretic dose is recommended.3,29 Multiple studies have demonstrated that low urinary sodium early after the first loop diuretic dose is associated with adverse outcomes, irrespective of fluid loss.30–32 However, interpreting spot urine sodium necessitates a comprehensive approach, considering its relationship with factors, such as diuretic dose, time gap between diuretic administration and urinary sodium measurement, the patient’s fluid volume status (the lower the volume, the lower sodium excretion, i.e., braking phenomenon) and neurohormonal activity.29 For instance, it is possible to encounter patients in whom urinary sodium decreases ‘physiologically’ in parallel with decongestion and others in whom it remains stable or even increases during the subsequent days of admission as a reflection of the persistence of a positive sodium balance.33
Haemoconcentration
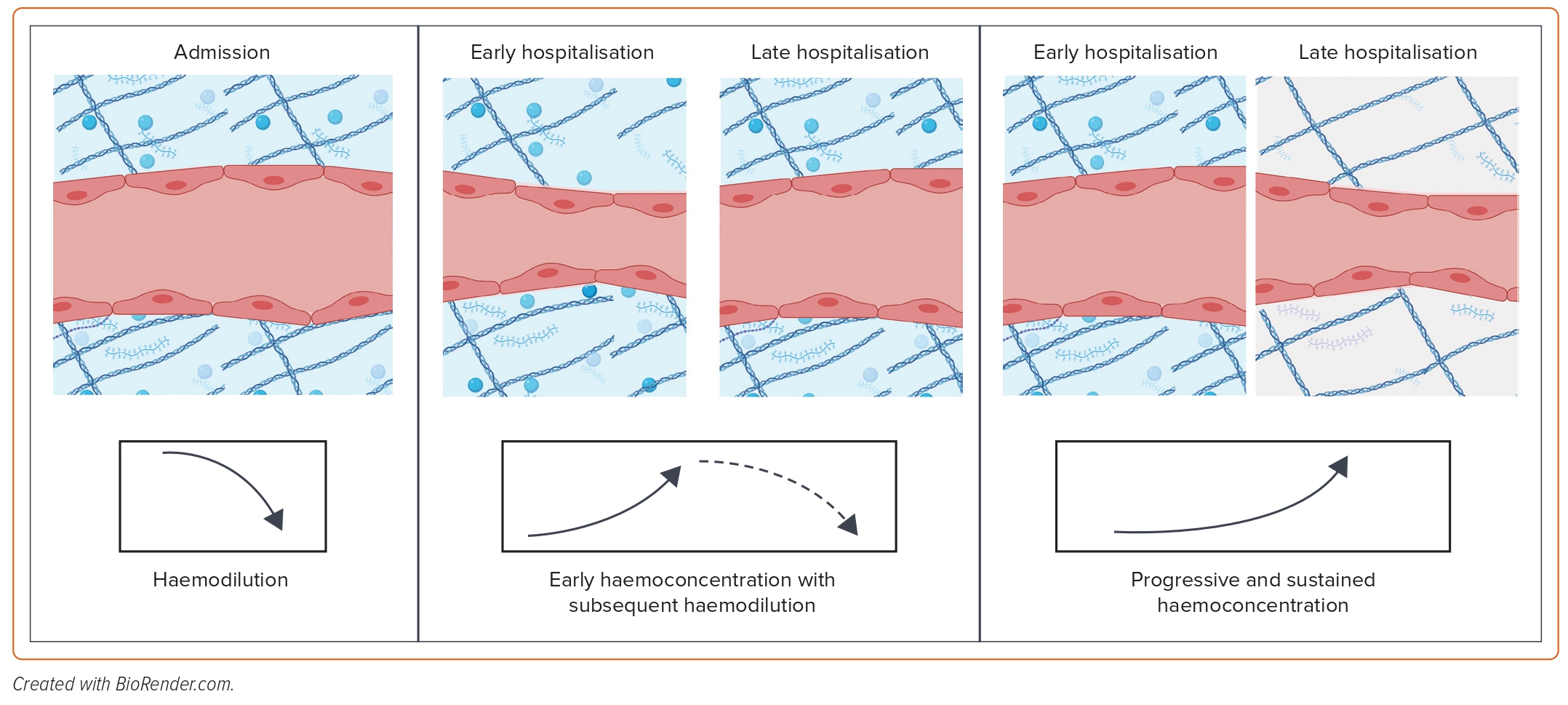
Haemoconcentration, which is the increase in the concentration of red blood cells and plasma proteins due to intravascular volume contraction, can be used as an indicator of effective intravascular decongestion.12 This process seems to identify patients who have undergone aggressive decongestion and has been associated with better post-discharge survival. However, since most of the excess volume in patients with AHF resides in the interstitial compartment, haemoconcentration may only indicate that the plasma refill rate has been exceeded even in the presence of extravascular volume expansion, which indicates the removal of intravascular fluid at a rate faster than it can be refilled from extravascular stores. As a result, haemoconcentration alone does not inform total body volume status. In fact, an early haemoconcentration with subsequent haemodilution is not associated with clinical benefit as it may indicate that diuretics may have been de-escalated too early before the complete elimination of extravascular volume expansion, resulting in the recurrence of intravascular volume overload – a rebound effect (Figure 3).34 Conversely, a late and sustained increase in haematocrit/haemoconcentration in parallel with the patient’s clinical improvement may be more informative about the equilibration of extravascular and intravascular volume (Figure 3).34
Biomarkers for Improving Recognition and Management of High-risk Patients at Discharge and During the Transitional Phase
The transitional phase following admission for AHF is a critical period in a patient’s care trajectory, requiring meticulous surveillance and management. During this period, which comprises the initial week post-discharge, patients have the highest risk for readmission and adverse outcomes.35 Multiple factors contribute to this heightened vulnerability, with residual congestion and inadequate post-discharge care coordination emerging as the most critical elements to address.
Several strategies have been proposed to reduce the burden of early post-discharge events on patients and healthcare systems, with a targeted approach being a potentially cost-effective solution.35 This strategy involves discharging low-risk patients with less intensive post-discharge monitoring and providing high-risk patients with comprehensive in-hospital and post-discharge care, potentially promoting a more efficient distribution of scarce healthcare resources. However, accurately identifying patient subgroups at varying risk levels and predicting events such as hospital readmissions remains a significant clinical challenge. Biomarkers can play an essential role as objective tools for short-term post-discharge risk stratification, and several promising prognostic biomarkers are currently available that might assist clinicians in assessing post-discharge risk prediction on top of traditional clinical models.
Natriuretic Peptides
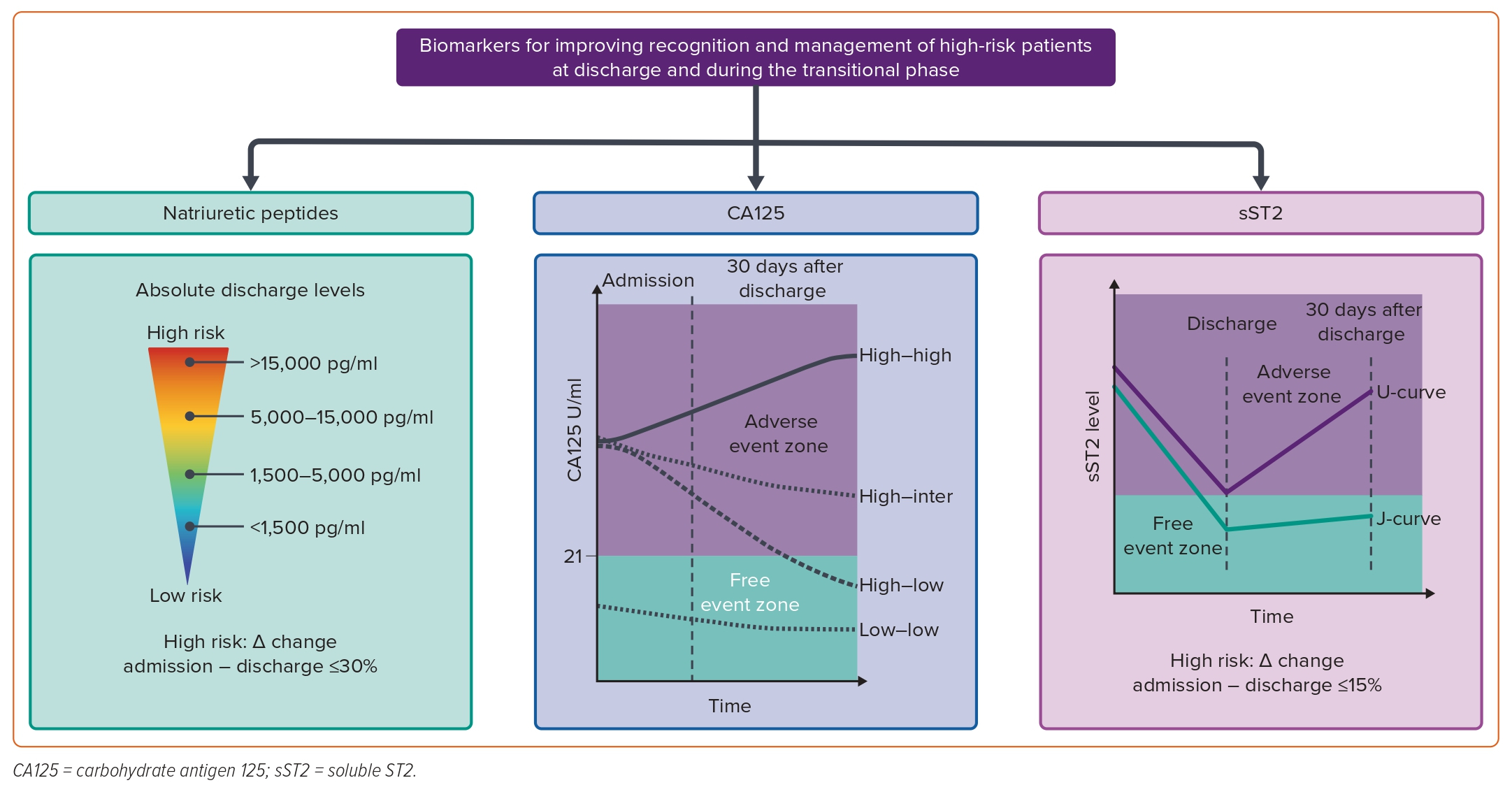
Although the NP concentration at admission for AHF strongly predicts inpatient mortality, the absolute discharge value and the percentage change in levels from admission to discharge seem to be better predictors of short-term post-discharge outcomes.36–39 For instance, an individual patient data meta-analysis of seven prospective cohort studies, including 1,301 patients discharged with NT-proBNP measurements available at admission and discharge, showed that the 180-day cumulative mortality rate varies significantly with different NT-proBNP absolute discharge levels: 4.1% for <1,500 pg/ml, 10% for 1,500–5,000 pg/ml, 24% for 5,001–15,000 pg/ml, and 41% for >15,000 pg/ml (Figure 4).38 A similar pattern was observed when using the reduction percentage, showing that patients with a ≤30% reduction in NT-proBNP levels have twice the 180-day cumulative mortality and composite event rate (all-cause mortality and/or first readmission for a cardiovascular reason) compared to those with a >30% reduction in NT-proBNP levels (Figure 4).38 Likewise, long-term repeated measurements following the index hospitalisation for AHF have also been shown to be independently associated with the risk of death.40 A descending kinetic pattern identified those at lower risk of death, although the ability of serial measurements to predict survival seems to be blunted at longer follow-up periods.40
While reductions in NT-proBNP levels generally mirror improved central cardiac haemodynamic and cardiomyocyte stress, indicating successful response to treatment, there are some clinical scenarios in which NP values do not significantly decline despite optimal treatment and warrant further evaluation.41 First, a persistently elevated absolute NP concentration at discharge in a euvolemic patient may represent their ‘dry’ NP level, identifying that they may have advanced disease, such as persistent cardiomyocyte stress.
Another possible scenario involves a patient with predominant right-sided HF and significant systemic congestion/third-space fluid accumulation who may need a longer diuretic period before achieving a steady decrease in NP levels.42 In this clinical setting, persistently elevated NP levels at discharge likely result from fluid mobilisation from the interstitial compartment to the central circulation, leading to a rebound increase in cardiac filling pressures once the parenteral diuretic therapy has been stopped. Finally, treatment does not effectively correct the central cardiac haemodynamics in some cases, which identifies a treatment-resistant, high-risk patient with a poor prognosis.
In summary, patients who experience a substantial decrease in NP levels after AHF treatment are more likely to have favourable early post-discharge outcomes and a less stringent follow-up may be deemed appropriate. On the other hand, those with higher or non-decreasing concentrations might require closer monitoring and advanced interventions. The evidence in patients with AHF and HFpEF and those with lower expected values on admission is scarce and requires further evaluation.
CA125
One of the most attractive properties of CA125 is its potential role in monitoring the clinical course following an AHF event.21 Small studies have indicated that changes in CA125 align with shifts in clinical status. Other studies have also demonstrated the usefulness of CA125 changes for risk stratification within the first week after discharge.40,43,44 In a cohort of 293 patients admitted for AHF, CA125 levels decreased to ≤35 U/ml in 52.2% of patients, decreased but remained >35 U/ml in 24.6%, and increased in 23.2% at the first outpatient visit after discharge (median 31 days).43 At a median follow-up of 18 months, patients with normalised CA125 values had the lowest risk of death.43 In contrast, those with decreased but not normalised values and those with increased values had a higher adjusted risk of death. Changes over time showed incremental reclassification indexes compared to a single measurement of CA125 or even changes in NT-proBNP.43 The same categorical changes proved to be useful for predicting 6-month readmission. Patients who did not have CA125 ≤35 U/ml at the first outpatient visit had a threefold increased risk of 6-month HF readmission.43
In a more recent study, the long-term changing patterns of CA125 were found to be independently associated with mortality.40 The study involved 946 consecutive patients discharged for AHF and assessed the long-term prognostic impact of repeated CA125 measurements (3,402 observations during an average follow-up of 2.64 years). The outcomes significantly differed based on survival status. A considerable decrease in CA125 levels was observed within the first month after discharge, which identified a lower-risk subgroup (Figure 4). In contrast, CA125 levels remained high or increased in patients who died at the end of follow-up (Figure 4).40 Moreover, longitudinal measurements of NT-proBNP were also linked to higher mortality risk. However, unlike the changes in CA125, the magnitude of these changes was less pronounced and diminished over time. Notably, a shift from high to low or low to high CA125 levels during follow-up was strongly correlated with a decreased or increased risk of death, respectively.40
The CHANCE-HF trial involved 380 patients discharged for AHF with high CA125 levels (>35 U/ml). This study compared a CA125-guided therapy to the standard of care.45 In brief, the CA125-guided therapy involved intensifying diuretic treatment and monitoring frequency when CA125 levels increased or remained high during follow-up.45 When CA125 levels decreased below 35 U/ml, the protocol recommended reducing diuretics and increasing the time between outpatient visits. Patients in the CA125-guided strategy experienced a 50% increase in diuretic dose modifications and up to 21% of them received outpatient IV furosemide based on persistently high CA125 levels.45 The guided strategy proved superior to the standard of care in reducing the risk of the composite 1-year death or AHF readmission. This reduction was achieved by decreasing the risk of the first and recurrent HF readmissions by 51% at the 1-year follow-up.45
In summary, current evidence supports the role of CA125 in dynamic risk stratification, which is independent and additive to the prognostic information provided by traditional proxies of HF severity and NPs.21 Patients with elevated CA125 require a comprehensive assessment of congestion and may benefit from intensive diuretic strategies accompanied by closer monitoring. Normalising CA125 values in this population should be the objective regardless of initial post-discharge values.21 Persistently high or non-normalising CA125 levels during the first week after discharge may indicate ongoing/residual congestion and an increased risk of adverse clinical events (Figure 4).21
Soluble ST2
ST2, also known as interleukin-1 receptor-like 1 (IL1RL1), belongs to the Toll-like/interleukin-1 receptor superfamily and is encoded by the IL1RL1 gene situated on chromosome 2.12 Alternative splicing generates multiple ST2 isoforms, including transmembrane (ST2 ligand or ST2L) and soluble circulating form (sST2). The latter is a valuable prognostic biomarker in both acute and chronic HF. The biological action of the ST2 protein is mediated through the extracellular engagement of ST2L with its ligand, interleukin-33 (IL-33). While the IL-33/ST2L signalling pathway has anti-fibrotic effects, sST2 acts as a decoy receptor, diminishing the net transduction of the favourable effects of IL-33 through ST2L.12
Single sST2 measurements have consistently demonstrated prognostic and predictive value, as confirmed in two meta-analyses conducted on chronic and acute HF.46,47 However, serial sST2 measurements more accurately reflect the dynamic and progressive nature of HF compared to a single measurement.48 For instance, Boisot et al. demonstrated that among patients hospitalised with AHF, a failure of sST2 to decrease by at least 15% during hospitalisation was associated with an increased risk of death at 90 days (Figure 4).49 Building on this concept, Bayés-Genís et al. measured sST2 2 weeks apart in a cohort of outpatients with decompensated HF and observed that these patients were at increased risk of adverse outcomes when sST2 failed to drop by at least 25%.50 More recently, van Vark et al. examined the predictive value of frequently measured sST2 in a population with AHF three times during admission and four more times after discharge at pre-defined time points.51 This study shows that baseline sST2 and repeated sST2 measurements represent a strong, independent predictor of the composite endpoint of all-cause mortality or readmission for HF during a 1-year follow-up. A U-shape sST2 pattern was observed in those with the primary endpoint – sST2 concentrations increasing before reaching the primary endpoint – whereas ST2 stabilised in patients without the primary endpoint during follow-up – a J-shape sST2 pattern (Figure 4).51
Although several new biomarkers also seem to be promising for risk stratification, such as growth differentiation factor-15 (GDF-15) and bioADM, optimising the assay methodology remains a major challenge. Furthermore, only a few new-generation HF biomarkers have fully automated protocols, while some do not even have Food and Drug Administration approval or a CE label.52
Multibiomarker Panel: Ready for the Prime Time?
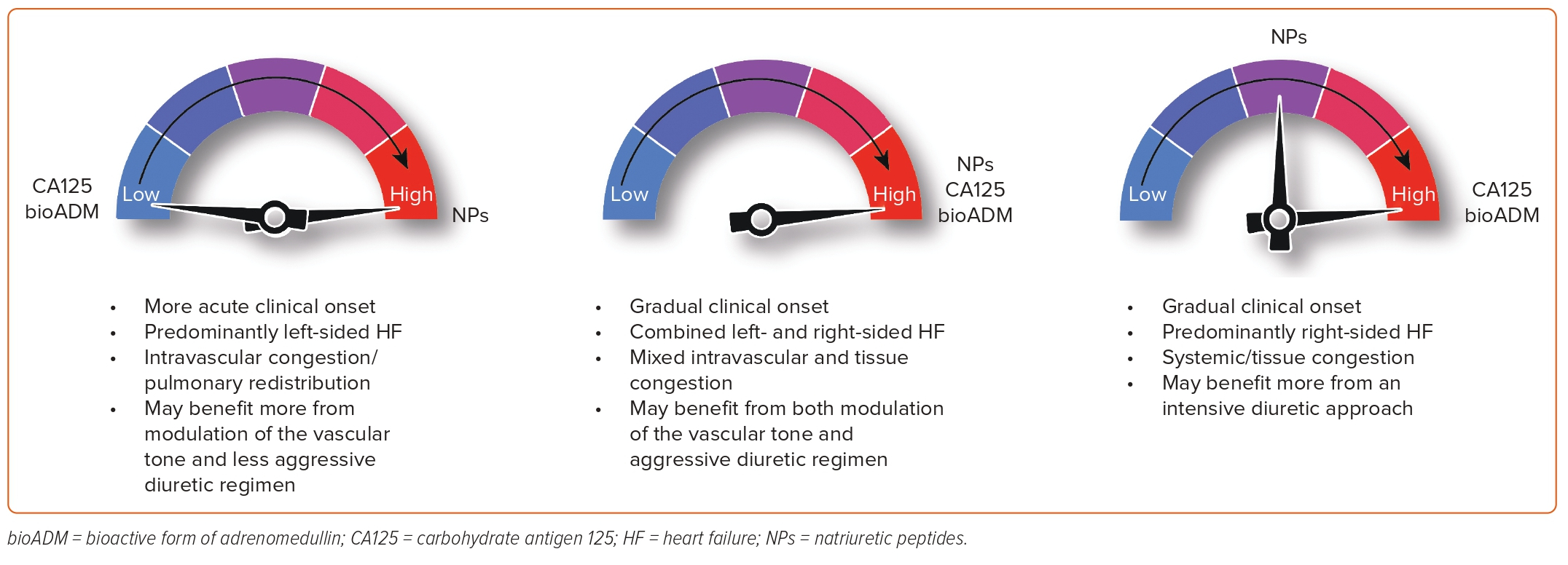
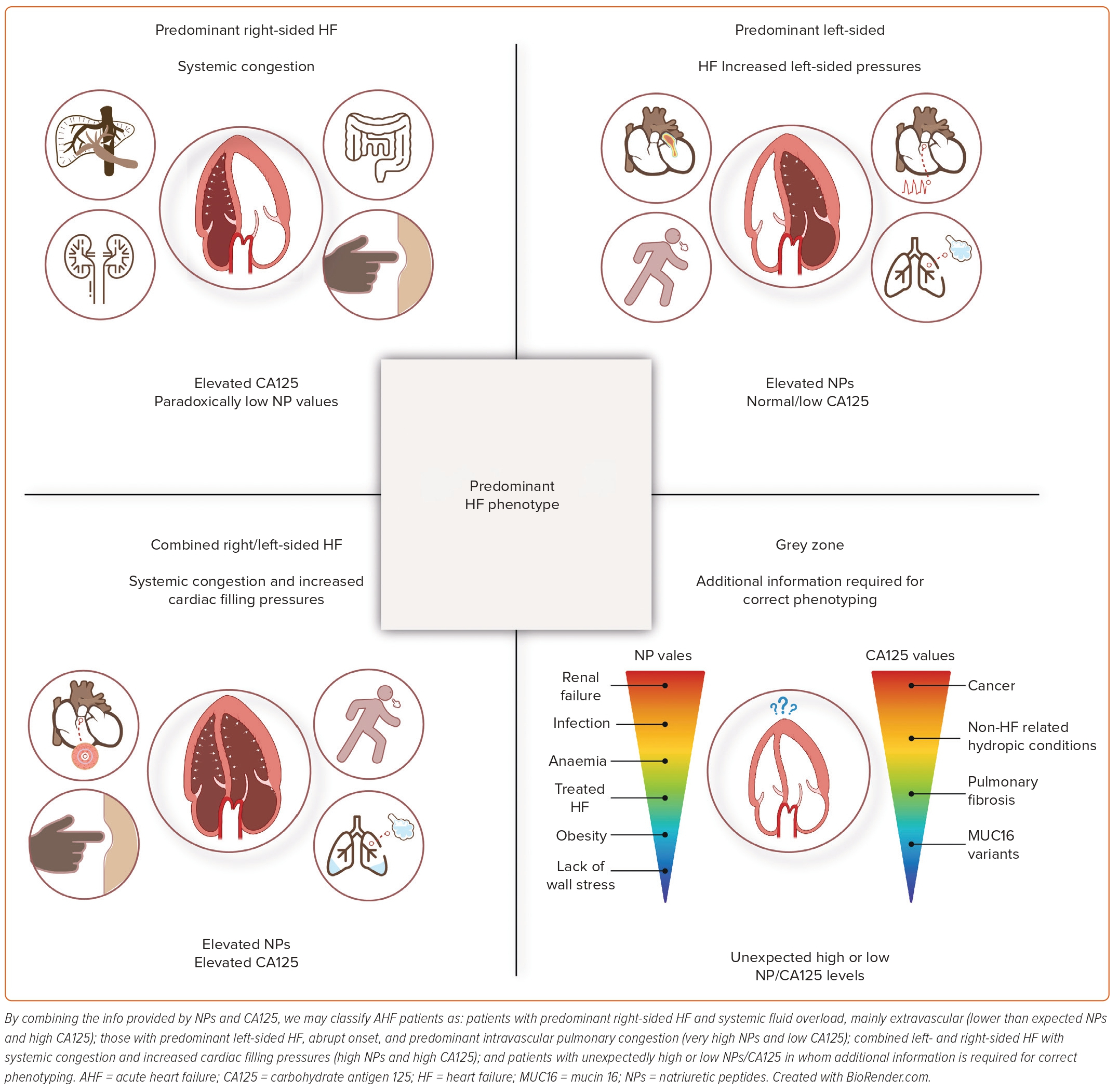
In our opinion, a multiparametric approach that considers the key pathophysiological mechanisms in AHF is highly relevant. This approach should be logistically feasible, widely available, relatively low-cost, highly reproducible and not excessively time-consuming. It should also provide additional information to the standard of care to improve diagnosis, monitoring and to guide therapy. In AHF, this panel should ideally include proxies of LV stretch and pressures (NPs) and information on the distribution of fluid overload, both regional and compartmental. CA125 appears to be an ideal candidate for assessing the type of fluid overload. By combining the information provided by NPs and CA125, we can classify AHF patients into four categories based on the predominant type of HF, the onset of symptoms and the type of fluid overload (Figure 5). The usefulness of this panel, in conjunction with imaging techniques, is attractive but requires further investigation.
Conclusion
Biomarkers in AHF management are critical for enhancing diagnostic accuracy, monitoring therapeutic response and assisting in risk stratification during inpatient stays and transitional phases. NPs provide valuable information on cardiac function and help guide diagnosis and prognostic evaluation in AHF patients. Other biomarkers, such as spot urine sodium and haemoconcentration, can help predict diuretic response and assess effective decongestion. However, due to the multi-layered complexity of HF, relying solely on a single biomarker strategy may not offer significant additional benefits. Instead, a multi-biomarker approach is likely to be necessary to reflect the entire disease burden. Therefore, we believe there is considerable scope for further research to elucidate whether these markers accurately mirror specific shifts in disease severity or comorbidities. In addition, novel biomarkers, such as CA125 and sST2, can offer additional prognostic information, especially when combined with NPs. Notably, a multiparametric approach incorporating key pathophysiological mechanisms in AHF and including a panel of biomarkers such as NPs and CA125 could help classify patients into distinct categories based on the predominant type of HF, symptom onset and fluid overload. This classification system could potentially improve diagnosis, monitoring, and therapy guidance in AHF patients. However, more research is needed to confirm the use of this panel in conjunction with imaging techniques in routine clinical practice.