Chronic heart failure (HF) is a major health problem that affects approximately 64 million people worldwide. It is estimated that 1–3% of the general population in developed countries lives with HF and one in five individuals will be diagnosed with HF at some point in their lives. The burden of HF increases with age: HF affects 10% of individuals >70 years old, and more than 80% of cases are diagnosed in people >65 years old.1,2
Higher baseline cardiovascular risk and lower mortality due to improved treatment of major cardiac and non-cardiac diseases are responsible for the progressive rise in the incidence of HF. The impact of HF on healthcare is also growing. HF is the first cause of admission among the older population, is a leading cause of death (3% of men and 10% of women die from HF) and consumes 1–3% of healthcare spending in developed countries.3–5
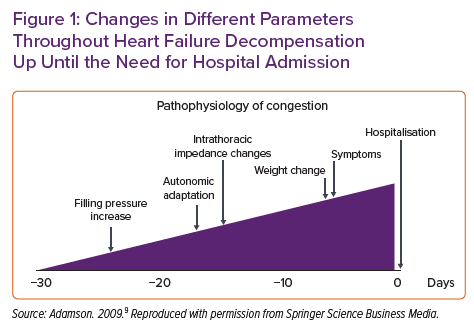
The natural history of HF is characterised by periods of stability interrupted by episodes of acute decompensation. Decompensation is defined as the clinical and haemodynamic deterioration caused by an increased cardiac filling pressure leading to systemic and/or pulmonary congestion and, in severe cases, peripheral hypoperfusion. Decompensation becomes more frequent as the disease progresses and is a marker of poor prognosis. In addition, most of episodes of decompensation require hospital admission, which is the main healthcare cost related to the disease.6–8 In this context, it is necessary to develop strategies to diagnose and treat episodes decompensation at early stages to prevent hospital admissions and other adverse events. This is not easy, because the symptoms and signs of decompensation usually appear late in the pathophysiological chain that leads to an HF decompensation (Figure 1).9
Since the early 2000s, several telemonitoring (TM) strategies have been developed in an attempt to detect preclinical deterioration in HF. A distinction can be made between non-invasive and invasive TM modalities, as discussed below.
Non-invasive Telemonitoring
Non-invasive TM primarily uses two strategies: structured periodic telephone calls conducted by a trained clinical team; and the automatic registration of symptoms and vital signs, such as blood pressure, heart rate, cardiac rhythm, peripheral saturation and weight using remotely connected medical devices. The first strategy is proactive because it requires contacting the patient directly. The second strategy allows passive monitoring, with no need for direct interaction if not indicated. The aim in both cases is the early detection of symptoms and signs of decompensation. Both strategies have been tested in several randomised clinical trials with conflicting results. Although these strategies improve self-care and adherence, they have not consistently been shown to reduce hospital admission and mortality in HF patients.10
Alternatively, percutaneous non-invasive devices that can measure the intrathoracic water in various ways as a surrogate of pulmonary congestion have been developed. Even though some of these devices showed promising results in observational studies, to date they also have failed to demonstrate a prognostic impact in randomised clinical trials.11–13
Invasive Telemonitoring Strategies
Invasive TM strategies require the implantation of intravascular devices, such as haemodynamic TM devices and cardiac implantable devices.
Haemodynamic TM devices directly measure the pressure of cardiac chambers or arteries using small manometers. Their potential usefulness is based on detecting the increase in intracardiac pressure that precedes an episode of HF decompensation. The most relevant advance in the field of haemodynamic TM has been CardioMEMS, a wireless device that monitors pulmonary artery pressure. In the CHAMPION-HF clinical trial, CardioMEMS was shown to reduce mortality and hospital admissions in patients with HF with reduced ejection fraction, New York Heart Association (NYHA) class III and a previous hospitalisation.14 However, these results could not be validated in a more heterogeneous cohort in the GUIDE-HF study.15 Devices implanted in other chambers, such as the left atrium or left ventricle, have not demonstrated efficacy in preventing episodes of decompensation or have presented an unacceptable incidence of procedure complications.16–18 Although more technologically advanced haemodynamic monitoring devices are in development, to the best of our knowledge none is currently under clinical evaluation.
With regard to cardiac implantable devices, additional functionalities have been developed to implement the follow-up of HF patients with reduced ejection fraction that carry an ICD, a cardiac resynchronisation therapy (CRT) defibrillator (CRT-D) or a CRT pacemaker (CRT-P). The first algorithms tried to associate one parameter, such as changes in heart rate, rhythm or thoracic impedance, with clinical worsening. One of the most well-known algorithms is Optivol (Medtronic), an index that quantifies daily changes in thoracic impedance as a surrogate for lung congestion. Although Optivol and some other one-parameter algorithms have been shown to improve the follow-up of HF patients, none has reduced hospitalisation or mortality in clinical trials.19–21 More complex detection systems, combining multiple variables as surrogates for volume status, have been developed in an attempt to impact HF prognosis.22–24 In the PARTNERS-HF observational study, an algorithm combining patient activity, heart rate, atrial fibrillation burden, Optivol index and proportion of CRT pacing proved to be useful in selecting patients with HF at higher risk of HF decompensation.25
In this paper, we focus on HeartLogic (Boston Scientific), a novel multiparametric algorithm that potentially will have a complementary role to guideline-directed management of patients with HF with reduced ejection fraction.
The HeartLogic Algorithm
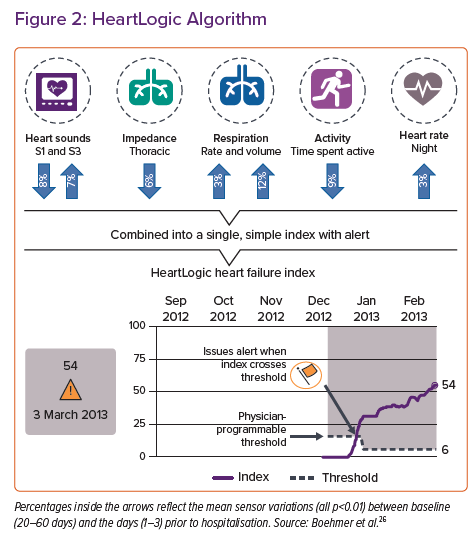
HeartLogic is a multiparametric algorithm implemented in certain ICDs (with or without CRT) from Boston Scientific, which allows stratification of the risk of decompensation in HF patients. HeartLogic is an automatic, remotely monitored system that combines trend analysis from different sensors implanted in the generator, namely nocturnal heart rate, intensity of the first and third sounds of the cardiac cycle, intrathoracic impedance, respiratory rate, tidal volume and physical activity, integrating them to generate a single numerical indicator, the HeartLogic index. When a patient is at risk of HF decompensation there will usually be a progressive rise in the heart rate and the intensity of the third sound, a decrease in the first sound of the cardiac cycle, breathing will become shallower and faster, diminishing inspiratory volume, pulmonary congestion will reduce intrathoracic impedance and physical activity will be limited by a deterioration in the functional class.26 It is through the combination of information from the different measures that the HeartLogic index estimates the risk of HF decompensation (Figure 2).
The HeartLogic index is specific for each patient and depends on the value of each parameter at steady state. Its lower value, called the baseline HeartLogic index, is calculated over a 3-month rolling window of each patient evolution. An increase in the index reflects a deviation from the baseline situation towards the decompensation in HF.
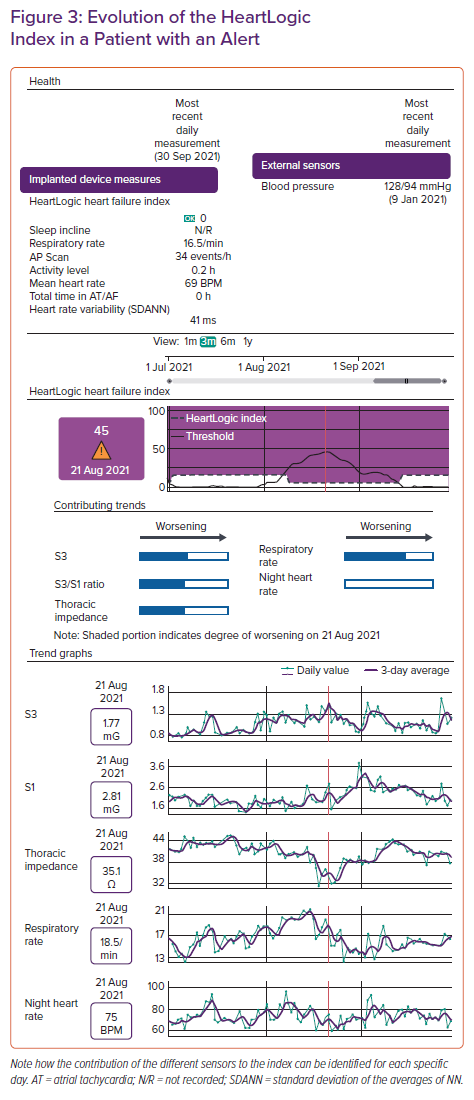
HeartLogic measurements of each parameter are automatically updated on a daily basis, as is the HeartLogic index. This makes it possible to describe a trend in the patient’s evolution that can be represented on a graph over time. The HeartLogic index remains stable if the clinical situation of the patient does not change acutely. When the HeartLogic index exceeds a prespecified numerical value, the device issues an alarm, the HeartLogic alert, indicating a higher risk of decompensation. This value is predefined at 16, because this value demonstrated the best sensitivity and specificity ratio for detecting an HF event in the MultiSENSE study, but it can be adjusted individually for each patient or group of patients.27 If the patient improves and approaches the baseline condition again, as may happen after a patient has been prescribed diuretics, the HeartLogic index decreases (Figure 3). When the index falls below 6, the alert will be resolved.
The information from the HeartLogic algorithm is transmitted via a communicator to the Latitude NXT system, a remote monitoring virtual platform that can be accessed by the medical team, allowing for the follow-up of multiple patients and their stratification according to the risk of decompensation. A patient will only be aware of a HeartLogic alert status if they are directly contacted by their healthcare professional. Of note, the HeartLogic has a minimal effect on the longevity of the on the ICD (±CRT). The reduction in battery durability with HeartLogic sensor data collection and daily alert checks is approximately 2 months.
Evidence
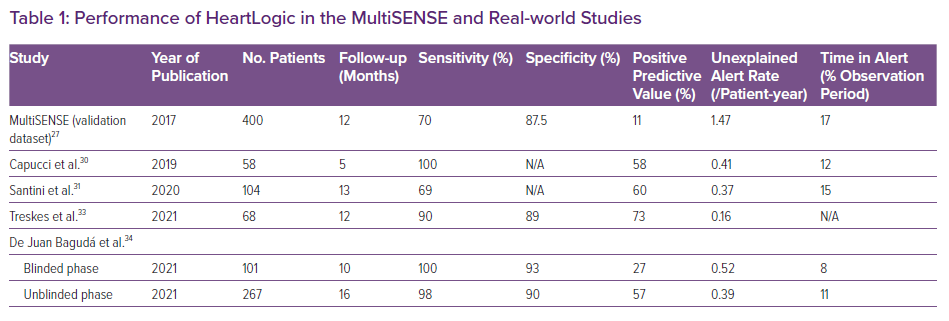
The usefulness of the HeartLogic algorithm has so far been evaluated in observational studies. The MultiSENSE study was the first to evaluate the usefulness of HeartLogic in predicting HF decompensation.27 That study included 900 patients with a COGNIS (Boston Scientific) CRT-D with HF with reduced ejection fraction, NYHA class II–IV or with an admission for HF or need for IV diuretic administration within the past 6 months. The study used one cohort for development of the HeartLogic algorithm and another for its validation. In the MultiSENSE study, the HeartLogic algorithm demonstrated a maximum sensitivity of 70% with an index value of 16 for predicting HF decompensation, defined as a hospital admission or unplanned visit requiring intravenous therapy. Its specificity was 86% and its negative predictive value was 99.9%. The median time from alert (exceeding an index value of 16) to HF decompensation was 34 days, and, of note, there was a low incidence of false alarms (1.47 per patient per year).27
The HeartLogic index not only changes throughout a patient’s evolution and predicts HF decompensation, as described above, but its baseline value also seems to select patients with a higher risk of decompensation. Patients that decompensate tend to have higher baseline HeartLogic index values than those who remain stable.28 In addition, the HeartLogic index has been shown to have a prognostic value independent of N-terminal pro B-type natriuretic peptide (NT-proBNP). An increase in the HeartLogic index above 16 is an indicator of risk of admission in patients with and without elevated NT-proBNP. Moreover, patients who are in alert and have elevated NT-proBNP seem to be at higher risk of decompensation.29 In a subanalysis of the MultiSENSE study, patients in the HeartLogic alert state had a 24- and 50-fold higher risk of HF decompensation by at the 12-month follow-up if their NT-proBNP concentrations were <1,000 pg/ml and >1,000 pg/ml, respectively, compared with patients with NT-proBNP <10,00 pg/ml and a negative HeartLogic index.29
Few observational studies have shown how HeartLogic performs in real-life clinical practice (Table 1). In general, these studies demonstrated that the algorithm allows the identification of relevant HF-related conditions with high sensitivity and specificity and enables effective clinical action to be taken remotely, with a low incidence of false alarms.30–32 One multicentre study compared 1-year HF hospitalisation before and after activation of the HeartLogic algorithm in 68 patients, showing a 66% reduction in HF admissions, a >50% decrease in the hospitalisation length of stay and a significant reduction in overall health economic costs after activation of the HeartLogic algorithm.33 In a prospective study in 366 ICD patients recruited from 22 centres, at the 11-month follow-up HeartLogic alerting was associated with a 24.5-fold increased risk of hospital admissions.32 In addition, in that study, alarms followed by a clinical intervention were associated with a lower incidence of events at follow-up.32 In another multicentre study conducted in 102 patients who were followed for a median of 13 months, 100 alerts were recorded, 60% of which were of clinical interest.31 The incidence of non-insignificant alerts has been reported to be only 0.37 per patient-year, and the rate of hospitalisations not preceded by an alert is very low (0.05 per patient-year).21,22
We have recently published an ambispective study with the first data from the RE-HEART registry, which included 288 patients from 15 centres across Spain.34 In that study, the HeartLogic algorithm was shown to predict a HF decompensation or clinically relevant event in more than half of the alerts, on average 20 days in advance. Sensitivity and negative predictive value were close to 100%, with a specificity of over 90%.34 The HeartLogic was demonstrated to be useful in identifying events and individualising patient follow-up, knowing that decompensation outside the alert state is unlikely. It is also of note that the reported rate of unexplained alerts was low (0.39 alerts per patient-year) and that in more than 80% of cases alerts could be resolved by telephone.34
However, these promising results have not yet been validated in a randomised study. MANAGE-HF (NCT03237858), currently in the inclusion phase, is the first randomised clinical trial designed to assess the impact of HeartLogic on hospitalisation and mortality in symptomatic (NYHA functional class II–III) HF patients with either a previous decompensation or elevated natriuretic peptides. It is planned that MANAGE-HF will include 2,700 patients with a Boston ICD, CRT-D or CRT-P who will be randomised 1:1 to a guideline-directed follow-up with HeartLogic monitoring on versus off. If the results of MANAGE-HF are positive, HeartLogic would be the first algorithm of implantable devices to demonstrate a prognostic impact in HF.
Application of HeartLogic in Daily Clinical Practice
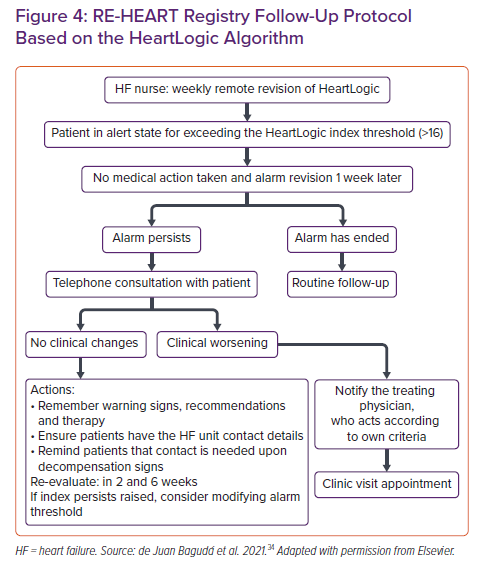
The HeartLogic algorithm allows daily TM of patients with HF and the detection of those at increased risk of decompensation. Its simplicity and the encouraging results of the observational studies described above have motivated its incorporation into the protocols of centres with HF units. Figure 4 shows the follow-up protocol based on monitoring with the HeartLogic algorithm in the centres included in the Spanish RE-HEART registry.34
When the HeartLogic index enters the alert state, it does so early enough to that the precipitating factors of an HF admission can be addressed. Through telematic or face-to-face contact three possible scenarios are defined:
- If the patient is symptomatic, medication can be adjusted, possible precipitating factors can be detected and corrected and early targeted treatment can be administered before the patient exhibits admission criteria. Subsequent follow-up can be done on an individual basis.
- If the patient is asymptomatic but has an active precipitating factor, the precipitating factor can be addressed and the response to the intervention can be assessed with individualised follow-up. Some precipitating factors are low adherence to medical treatment or dietary restrictions, the use of deleterious medications, such as non-steroidal anti-inflammatory drugs, the incidence of arrhythmias, such as AF and the loss of cardiac resynchronisation.
- If the patient is asymptomatic and no precipitating factor is evident, considering that this is a higher-risk patient, closer remote follow-up can be performed.
In our experience, the threshold value of 16 for the HeartLogic alert performs well in most patients and its individual adjustment is rarely needed. However, we have seen a small group of patients with a worse prognostic profile who are persistently above this value, like those with persistent congestion, diuretic resistance, low adherence to treatment or in advanced HF; in such patients, raising the alert threshold could be considered. Other very rare conditions could be patients with HF events but whose HeartLogic index does not reach the in-alert status (false negatives), for whom a lower threshold value would make sense.
Most HeartLogic alerts can be resolved via telephone by trained nursing staff with low time consumption. In the RE-HEART registry, only 60 minutes of clinical care time per week was consumed for every 30 patients monitored with HeartLogic.34
HeartLogic also selects low-risk patients based on its high negative predictive value. These patients are defined as those with a stable and negative HeartLogic index. In this case, the algorithm makes follow-up more flexible, minimising in-clinic contact with the healthcare environment. This factor is particularly relevant in the current epidemiological context, influenced by the COVID-19 pandemic.
Among the limitations of the HeartLogic system, it should be noted that this system only allows the monitoring of patients with a Boston Scientific ICD. Implantation is due to the presence of specific indications in HF, and therefore excludes many patients with a diagnosis of HF without indication for this therapy, such as those with a left ventricular ejection fraction >35% or those in NYHA functional class I. Furthermore, although the HeartLogic system has been shown to select patients at higher risk, it is not yet known whether it has an effect on hard clinical events, such as hospital readmission or mortality in the demanding setting of HF with reduced ejection fraction under optimal prognostic treatment. The MANAGE-HF clinical trial will resolve this issue. Finally, although initial data are promising, the role of the HeartLogic system in reducing direct and indirect costs in the follow-up of HF needs to be further evaluated.
Conclusion
HeartLogic seems to be a useful tool in the daily practice of an HF program. It can help to both identify patients at increased risk of decompensation, to enable prompt action to try to avoid an impending decompensation, and reassure patients out of alert, who can be followed in a more lenient way. Most patients can be managed by telephone, thus avoiding unnecessary visits to the clinic, improving patient experience. This proactive way of following patients is promising and will hopefully lead to improvements in the prognosis of HF.