Heart failure (HF) is defined as “a complex clinical syndrome that can result from any structural or functional cardiac disorder that impairs the ability of the ventricle to fill or eject blood”.1 Hyperkalaemia is commonly encountered in HF patients. Registry data estimate the prevalence to be between 16% and 25%.2,3 The definition of hyperkalaemia varies, but has generally been defined as a potassium level >5 mEq/l.4 It can be further subclassified into mild (5–5.5 mEq/l), moderate (5.5–6 mEq/l) and severe (>6 mEq/l).1
Hyperkalaemia poses a major obstacle in the management of HF patients. It can result in electrical issues from conduction system abnormalities to life-threatening rhythm disorders and interference with defibrillator function.5 It can also lead to the downtitration and discontinuation of renin–angiotensin–aldosterone system inhibitors (RAASi) and angiotensin–neprilysin inhibitors, which are the cornerstones of HF therapy.6,7 Furthermore, hyperkalaemia is a marker associated with an increase in hospital length of stay and mortality.8
Pathophysiology of Hyperkalaemia
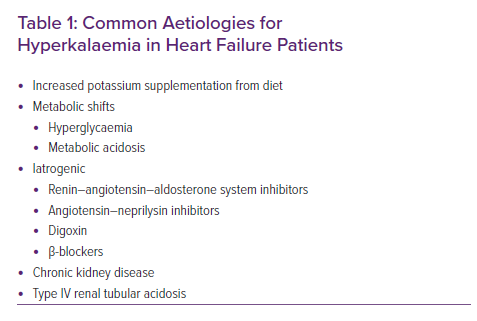
Hyperkalaemia occurs due to decreased excretion or increased intake of potassium (Table 1). Potassium levels are tightly regulated in the body; under normal circumstances, 98% of potassium is intracellular, with 2% being extracellular in the plasma.9 Approximately 90% of the potassium filtered through the glomerulus will be reabsorbed in the proximal tubule and the loop of Henle, and 10% will reach the distal tubules.9 During times of renal hypoperfusion, such as in HF, renin is secreted by the juxtaglomerular cells in the kidneys; this allows angiotensinogen to be cleaved to angiotensin I, which is then converted to angiotensin II in the endothelial cells of the lungs under the influence of angiotensin-converting enzyme (ACE).10 Angiotensin II acts on the cells of the zona glomerulosa to secrete aldosterone, which in turn promotes sodium and water retention.10 Aldosterone activates the Na+/K+ pumps in the basolateral membrane of the distal tubule and collecting ducts, creating a concentration gradient that results in the reabsorption of sodium and secretion of potassium ions. It also upregulates the epithelial sodium channels and potassium efflux at the luminal surface leading to further reabsorption of sodium and excretion of potassium.10
Patients with HF often have other comorbidities, including diabetes, chronic kidney disease (CKD) and hypertension.1 Therefore, the aetiology of hyperkalaemia in patients with HF can be multifactorial, as seen in acute decompensated HF, cardiogenic shock and chronic HF.11,12 Kidney injury, which may be in the setting of cardiorenal syndrome (type I and type II), results in hyperkalaemia due to reduced renal potassium excretion.11 Some diabetic patients can develop a type IV renal tubular acidosis that can directly result in hyperkalaemia.10
In clinical practice, medications are the most common cause of hyperkalaemia in HF patients. ACE inhibitors (ACEi) and angiotensin receptor blockers (ARB) decrease the production of aldosterone in the adrenal gland, while mineralocorticoid receptor antagonists (MRA) block the receptor.13,14
Incidence and Significance of Hyperkalaemia in the Heart Failure Population
Clinical trials involving RAASi have demonstrated varying rates of hyperkalaemia due to variability in the definition of hyperkalaemia used. In SOLVD, there was a 7.8% occurrence of hyperkalaemia (defined as a potassium level >5.5 mEq/l) in the enalapril arm compared with placebo.15 In CHARM, the incidence of hyperkalaemia was 5.2%.16 In PARADIGM-HF, the incidence of hyperkalaemia (potassium level >5.5 mEq/l) was similar between the sacubitril/valsartan and the enalapril arms, but the incidence of severe hyperkalaemia was lower in the sacubitril/valsartan arm (4.3% versus 5.6%).17 Interestingly, a follow-up analysis of PARADIGM-HF showed that sacubitril/valsartan had no effect on potassium levels overall, and in fact, those on therapy had a slight reduction in potassium levels compared with enalapril, especially when the latter was combined with an MRA.18
In EMPHASIS-HF, hyperkalaemia (defined as a potassium level >5.5 mEq/l) occurred in 11.8% of patients receiving eplerenone, whereas serious hyperkalaemia (potassium level >6 mEq/l) occurred in 2.5% of patients in the eplerenone group.19 In RALES, where an ACEi was combined with spironolactone, the incidence of hyperkalaemia (potassium level >5.5 mEq/l) was 13%, whereas serious hyperkalaemia (potassium level >6 mEq/l) was seen in only 2% of patients.20 Following the publication of RALES, data from Ontario, Canada showed a higher incidence of serious hyperkalaemia and higher rates of hospitalisation and in-hospital mortality. Those findings were later attributed to the widespread inappropriate prescription of MRAs.21,22
More recently, in January 2020, Rossignol et al. used the EPHESUS data to develop a risk prediction score that was then validated in the EMPHASIS cohort.23 The risk model incorporated multiple variables, including MRA use and time-dependent potassium levels. They found this model to be better than the MAGGIC score to describe the risk of cardiovascular events.23
A strong relationship between potassium levels and mortality in patients with chronic HF has been demonstrated in multiple trials.3,24,25 This may lead the clinician to consider withdrawal of therapies with proven mortality benefit, which may also lead to higher mortality.3
Beusekamp et al. analysed patients in the PROTECT trial to look for associations between hyperkalaemia and mortality in patients admitted for HF exacerbations.26 Potassium levels were followed daily until discharge or day 7 of admission. RAASi use was common, with 76% of patients on an ACEi and 46% on an MRA. Incident hyperkalaemia (defined as any potassium level >5 mEq/l) was seen in 35% of patients. Although incident hyperkalaemia was not associated with increased mortality at 180 days, it was associated with downtitration or even discontinuation of RAASi, especially MRAs, a variable that was independently associated with increased mortality.26
These findings are further supported by real-world data published from a large observational cohort of more than 114,000 patients (most had CKD and 13,113 had HF), where potassium levels >5 mEq/l led to downtitration of RAASi. This change was independently associated with major fatal and non-fatal cardiac events, as well as overall mortality.27 For MRAs in particular, registry analyses show that many other factors are associated with their underutilisation, including CKD, higher ejection fraction, male sex, older age and lower income.28
A Swedish nationwide registry found that the association between potassium levels and mortality in HF patients was U-shaped, and suggested that the optimal potassium level in HF patients is 4.2 mEq/l, with higher and lower levels associated with higher mortality.29 Interestingly, another analysis of the Swedish registry noted that the incidence of hyperkalaemia was higher in patients with HF with preserved ejection fraction, including severe hyperkalaemia.30 The 2016 European Society of Cardiology Guidelines recommend short-term cessation of RAASi and MRAs to manage acute hyperkalaemia, followed by careful reintroduction of the discontinued drug(s).4 These guidelines were published prior to the trials discussed above.
Acute Management of Hyperkalaemia
The most dreaded consequence of hyperkalaemia is the development of electrical abnormalities.31 As such, it is of paramount importance to stabilise the myocardial membrane with intravenous calcium, either in the form of calcium gluconate or calcium chloride, both of which act within 5 minutes.31 Potential side-effects include vasodilation-related hypotension and bradycardia. Calcium itself does not lower potassium levels in the bloodstream.31 Calcium chloride contains three times more elemental calcium than calcium gluconate. Therefore, in acute situations, calcium chloride is preferred. However, in less critical cases, the preferred agent is the gluconate salt, because it is less likely to cause tissue necrosis if it extravasates.32
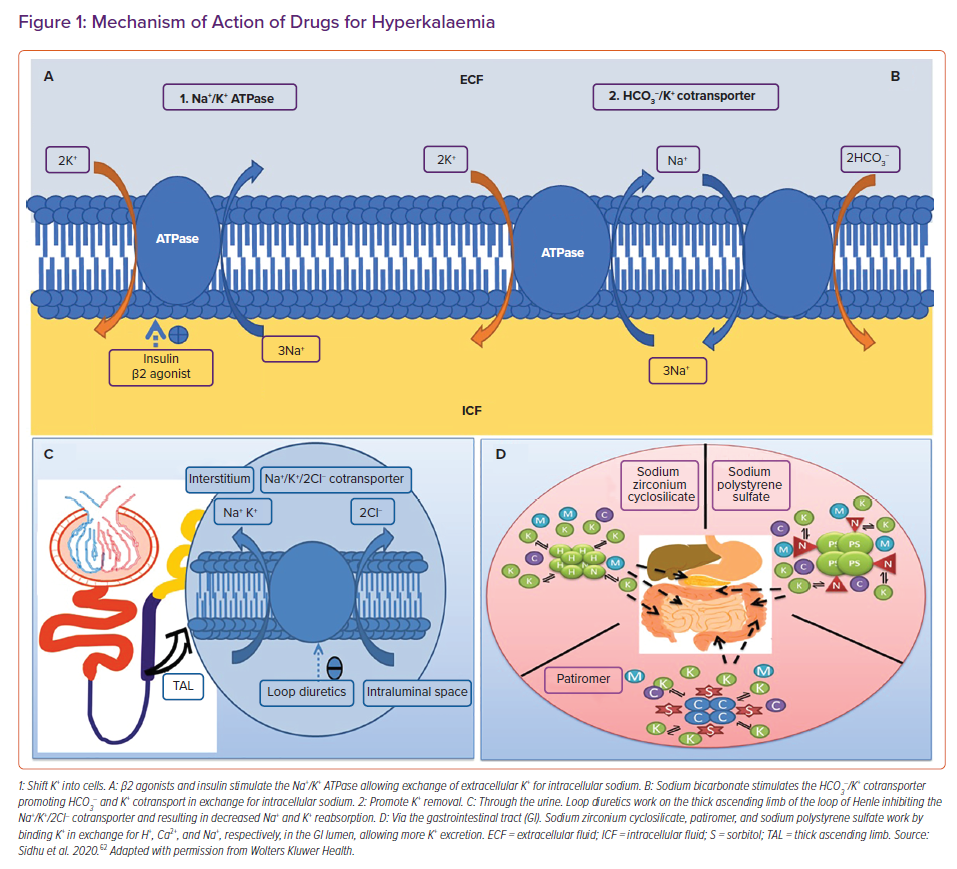
To acutely lower the potassium levels in the blood, agents that shift potassium from the extracellular to the intracellular space are utilised (Figure 1A). The most commonly used agents are short-acting insulins in combination with 25–50g of dextrose (unless hyperglycaemic). Insulin shifts potassium into the intracellular compartment via the action of the Na+/K+ ATPase pump. It works rapidly within 15 minutes. β2 agonists work similarly. Salbutamol is usually given at a dose of 10 mg and acts within 15–30 minutes. Side-effects include tachycardia, anxiety and headaches. Sodium bicarbonate can also be considered if the acutely hyperkalaemic patient is acidotic.33
After stabilisation, the next step is elimination. This is typically accomplished with loop diuretics, commonly furosemide. Furosemide works by inhibiting the Na+/K+/2Cl– cotransporter in the loop of Henle, and thus blocking sodium and potassium reabsorption. Increased sodium delivery to the distal nephron stimulates potassium excretion. Loop diuretics can precipitate hypokalaemia and are typically reserved for hyperkalaemic patients who are volume overloaded. Dialysis is recommended in cases of hyperkalaemia refractory to medical management (Figures 1B and 1C).34
The final step of hyperkalaemia management is maintenance. This is usually achieved on a long-term basis by eliminating the precipitants, minimising intake in the diet and reducing supplementation. Finally, binding resins can also be used if recurrent hyperkalaemia becomes an issue.5,35
Chronic Management of Hyperkalaemia in HF
Sodium Polystyrene Sulfate
Sodium polystyrene sulfate (SPS) is the oldest binding resin available. It is a non-specific binder that works to bind potassium ions in the gut in exchange for sodium (Figure 1D). As it is non-specific, it can also bind to calcium and magnesium. The potassium-bound SPS is eventually eliminated from the body in the faeces.36 The initial evidence for the use of SPS, culminating in its approval in hyperkalaemia, comes from two small trials published in the early 1960s.37,38
SPS is combined with sorbitol to relieve its constipating effects. It can be administered either as an oral (15 g 1–4 times/day) or rectal formulation (30–50 g every 6 hours). SPS use for hyperkalaemia is hampered by the fact that it has not been studied in large randomised controlled trials.39 One small trial of 33 outpatients with CKD evaluated the effect of SPS on mild hyperkalaemia (5–5.9 mmol/l) at a dose of 30 g for 7 days. The average reduction in potassium was 1.25 mEq/l. Normokalaemia was achieved in 73% of the patients on SPS.40
The main side-effects of SPS are gastrointestinal (GI), and include nausea, vomiting, constipation and diarrhoea. Serious side-effects have been reported with SPS, including colonic perforation and necrosis.41,42 This has been mainly attributed to the sorbitol added to SPS, which led to a black box warning in 2009 by the Food and Drug Administration. More relevant to a HF population, SPS can lead to worsening HF symptoms, as it exchanges potassium ions for sodium ions.43
Sodium Zirconium Cyclosilicate
Sodium zirconium cyclosilicate (SZC) is a novel binding resin. It is a non-absorbable, inorganic polymer of zirconium silicate, with the ion channels formed by its atomic structure mimicking those of endogenous potassium channels.44 It preferentially captures potassium ions, in exchange for hydrogen and sodium ions, through a selectivity filter (Figure 1D).
SZC works in the entirety of the GI tract; is not systemically absorbed and is excreted in the faeces. In vitro studies have shown that SZC can achieve a potassium equilibrium in as early as 20 minutes.45 As such, it can be used for acute (10 mg orally three-times a day for up to 48 hours) and chronic hyperkalaemia (5–15 mg orally daily).
SZC demonstrated significant efficacy in reducing K+ levels when used for hyperkalaemia in an emergency department setting compared with placebo, regardless of aetiology.46 When used as maintenance for chronic hyperkalaemia, SZC can be continued as long as it is clinically indicated.44 As it is not systemically absorbed following oral administration, SZC is thought to be safe in pregnant and lactating women.47
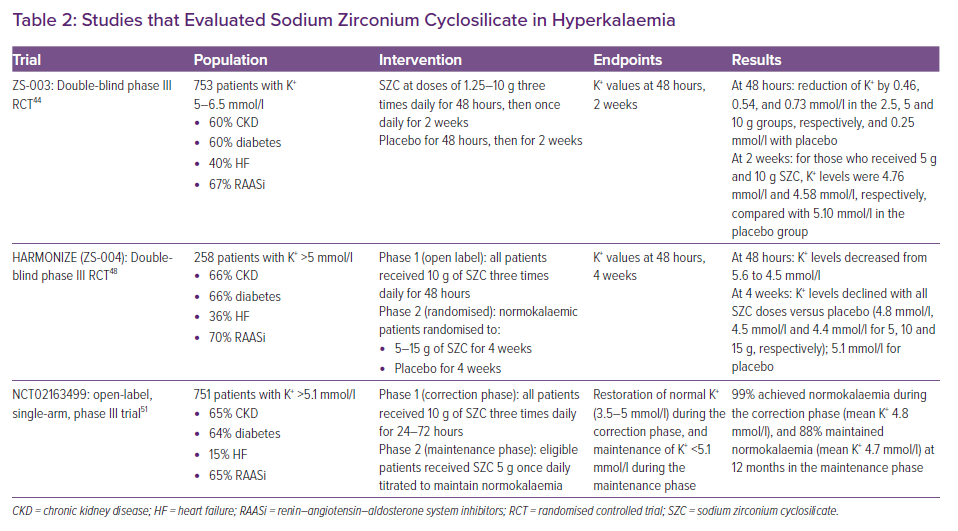
ZS-003 was a phase III trial that randomised 753 hyperkalaemic patients to receive SZC or placebo three times daily for 48 hours (see Table 2).44 Patients who achieved normokalaemia were then reassigned to receive placebo or SZC once daily for 2 weeks. Serum potassium was significantly lower in patients receiving the 5 and 10 g doses of SZC compared with placebo. The most common side-effects were GI related and occurred in 12.9% of patients on SZC.
HARMONIZE enrolled 258 ambulatory patients with hyperkalaemia. In the first 48 hours, they received 10 g SZC three-times a day (see Table 2). Nearly all were normokalaemic at 48 hours.48 This was followed by a maintenance phase, in which patients who achieved normokalaemia were randomised to receive SZC or placebo daily for 28 days. Normokalaemia was higher in all treatment groups versus placebo. Oedema was more common in SZC (2–6%). GI side-effects occurred in 2–9% of the patients on SZC, and these were dose dependent. HARMONIZE-Global was a follow-up trial that enrolled patients with a more diverse geographical distribution. Results were congruent with HARMONIZE, with 77.3% of the SZC group achieving normokalaemia compared with only 24% in the placebo group.49 In the subgroup of HF patients (n=94) from HARMONIZE, all three doses of SZC were effective in lowering and maintaining normokalaemia, including in those taking RAASi (n=60) .50
More recently, Spinowitz et al. assessed SZC in a single-arm study. SZC was able to acutely achieve normokalaemia during the first 72 hours of administration in nearly all patients (see Table 2).51 Following a 12-month maintenance period, 88% were normokalaemic and this enabled the initiation and/or uptitration of RAASi.51
Patiromer Sorbitex Calcium
Patiromer sorbitex calcium (patiromer) is a 100 µm bead organic polymer that preferentially uses calcium ions to exchange potassium ions (Figure 1D), although it can bind to magnesium and sodium. Patiromer is not systemically absorbed and is excreted in the faeces.52 It can be given as a starting dose of 8.4 g, and is increased in 4.2 g increments up to a dose of 25.2 g. It has a slow onset of action of approximately 7 hours, and therefore is only approved for chronic hyperkalaemia. Patiromer can be continued as long as it is clinically indicated.53 As it is not systemically absorbed following oral administration, SZC is thought to be safe in pregnant and lactating women.54
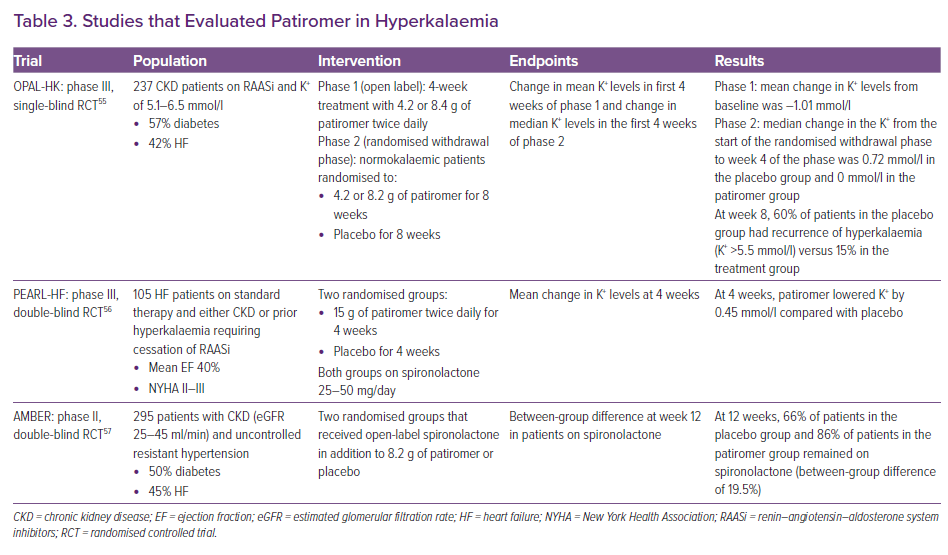
OPAL-HK was a study of 237 CKD patients with hyperkalaemia who were on RAASi (see Table 3).55 The study was divided into two phases: an initial 4-week phase where everyone received patiromer twice daily, followed by an 8-week withdrawal phase, in which patients were randomly assigned to continue the initial dose or placebo. By the end of the first phase, 76% of the study population were normokalaemic. During the withdrawal phase, there was a 15% incidence of hyperkalaemia in the treatment group compared with 60% in the placebo group. In the placebo group, 50% of patients were taken off RAASi in the withdrawal phase, whereas this occurred in only 6% of the patients in the patiromer group. The reported side-effects in this trial were constipation (11%), diarrhoea (8%), hypomagnesemia (8%), and hypokalaemia (3%).
A subgroup analysis of the OPAL-HK trial considered the effects of patiromer in HF (ejection fraction <35%) patients. Of the 102 patients with HF (42% of the study population), 76% were normokalaemic after the first 4 weeks. In the withdrawal phase, hyperkalaemia occurred in 52% of patients taking placebo and 8% of those on patiromer.55
PEARL-HF studied patiromer in patients with chronic HF (see Table 3). Patients included in the study either had stage III CKD or a history of hyperkalaemia necessitating the discontinuation of RAASi.56 A total of 105 patients were initiated on 25 mg/day of spironolactone, and were randomised to treatment with patiromer or placebo for 4 weeks. At 4 weeks, 92.7% of the patients in the patiromer group achieved normokalaemia compared with 75.5% in the placebo group. The patiromer group was more likely to have had spironolactone increased to 50 mg/day (91% versus 74%). Patiromer resulted in a 6% incidence of hypokalaemia in the trial, whereas hypomagnesemia occurred in 24% of the patients.
AMBER evaluated the effect of patiromer versus placebo on the rates of discontinuation of spironolactone in 295 patients with stage IIIb CKD and resistant hypertension over a 12-week period (see Table 3). All patients initially had a potassium level of <5.1 mmol/l, and were on either ACEi or ARB at baseline and were initiated on spironolactone. At the end of the 12 weeks, 86% of the patients in the patiromer arm remained on spironolactone compared with 66% of the placebo group. In the subgroup analysis of the patients with HF in the study (45% of the population), 84.1% of HF patients in the patiromer group remained on spironolactone compared with 68.1% in the placebo group. Hyperkalaemia was the most common cause of discontinuation, occurring in 23% of the placebo group versus 7% in the spironolactone group.57
Economic Impact
Hyperkalaemia poses a significant financial burden on the healthcare system. Mu et al. analysed a 5% random sample of Medicare beneficiaries in the US between 2010 and 2014 and randomised them based on the presence of hyperkalaemia (potassium level >5 mEq/l).58 Those with hyperkalaemia had more inpatient admissions, outpatient and emergency department visits and skilled nursing care facility admissions. Patients with hyperkalaemia and concomitant HF and/or CKD had even higher rates of hospital visits and longer inpatient stays. The subgroup of patients with HF and hyperkalaemia incurred a significant cost to the healthcare system at an average of US$11,750 over 30 days and US$49,244 over 1 year per patient compared with US$2,600 and US$23,936 in their normokalaemic counterparts.
Newer agents for hyperkalaemia remain expensive, but may overall save costs to the healthcare system. SZC 10 mg is US$749 for a 30-day supply, whereas patiromer 8.4 g is US$947 per month.59,60 In the British National Formulary, these prices are £427.20 and £172.50, respectively.61
Conclusion
Hyperkalaemia is a frequent issue encountered in patients with HF and is associated with increased mortality. RAASi interfere with potassium homeostasis, resulting in hyperkalaemia and lead to dose reduction or discontinuation of these agents, which have proven mortality benefits.
The novel potassium binding resins, SZC and patiromer, have shown positive short- and long-term results in counteracting hyperkalaemia and maintaining normokalaemia in various populations, including HF patients, and thus may aid in the optimisation of RAASi therapy and long-term maintenance.