Heart failure (HF) is a rapidly growing public health problem with an estimated prevalence of more than 26 million people worldwide.1 In developed countries the prevalence is 1–2 % peaking at ≥10 % among people aged over 70 years.2 In the US, the lifetime risk of developing HF is 20 % among people aged 40 years or older.3 Diagnosing the underlying cause of HF is central to the choice of appropriate treatment. Significant valvular heart disease (VHD; moderate and severe) was found in 14 % of patients who were referred for echocardiography due to suspected HF.4 Among patients with moderate and severe native VHD included in the Euro Heart Survey, 69.8 % presented with HF symptoms and the most frequent valvular lesions were aortic stenosis (AS) and mitral regurgitation (MR).5
Cardiac imaging plays a central role in determining the mechanism and the severity of VHD as well as the degree of accompanying left ventricular (LV) remodelling and systolic dysfunction. The primary dilemma for patients with VHD and HF is to determine whether the LV dysfunction is due to the disease of the valve or the ventricle. In patients with AS and HF symptoms, LV systolic dysfunction is usually secondary to the valve disease, while in patients with HF and functional MR, LV systolic dysfunction and remodelling are primary and are responsible for mitral valve malcoaptation. Furthermore, LV dimensions and ejection fraction (LVEF) are key parameters to indicate the need for valve surgery.6–8 With advances in percutaneous valve interventions – transcatheter aortic valve replacement (TAVR) and percutaneous transcatheter mitral valve repair, several other imaging parameters need to be evaluated to assess feasibility and predict therapeutic success. Echocardiography is the primary imaging modality and may be complemented by cardiac CT and cardiovascular magnetic resonance (CMR) when additional anatomical or functional information is needed. This review article focuses on the use of multimodality imaging to evaluate patients with HF and the most prevalent VHD – MR and AS – and how to decide the optimal intervention.
Mitral Regurgitation in Heart Failure
Significant (moderate and severe) MR is among the most common VHD, with an estimated prevalence of 1.7 % in the US peaking at 9.3 % in people older than 75 years of age.9 In one study involving 70,043 patients with suspected HF referred for echocardiography, MR of any severity was found in 12.5 % and moderate or severe MR in 3.1 % of patients.4 MR is classified as primary (organic) if there is primary structural abnormality of any component of the mitral valve apparatus (leaflets, chordae tendineae, papillary muscles or mitral annulus). The most common aetiologies include degenerative disease, rheumatic disease and endocarditis.10,11 In contrast, secondary (functional) MR results from LV dilation and dysfunction whereas the components of the mitral valve were originally normal. The main causes of secondary MR are ischaemic heart disease and dilated cardiomyopathy.10,11
Patients with severe primary MR commonly present with no or minimal symptoms.12 In contrast, HF is always present in secondary MR.13 In a large retrospective study including 1,256 patients with ischaemic and non-ischaemic cardiomyopathy, any grade of secondary MR was present in 73 % and 24 % had severe MR.13
Patients with HF and significant MR are usually evaluated using transthoracic and transoesophageal echocardiography. The underlying mechanism (primary versus secondary) and the severity of MR are systematically analysed. Grading of MR is based on a multiparametric approach which includes qualitative, semi-quantitative and quantitative parameters (Table 1).6,10 It is important to note that the evaluation of MR severity is significantly influenced by the LV loading conditions and the systemic blood pressure.14 In people with HF, decreased transmitral pressure gradients – due to lower systemic blood pressure and high left atrial (LA) pressures – result in lower velocity regurgitant jets, which appear small on Doppler colour flow images.10 Furthermore, vena contracta and flow convergence assume circular geometry at the regurgitant jet orifice. In secondary MR, the regurgitant orifice is frequently crescent in shape, and vena contracta, regurgitant volume and effective regurgitant orifice area (EROA) calculated using the proximal isovelocity surface area (PISA) method may therefore significantly underestimate the severity of MR.10
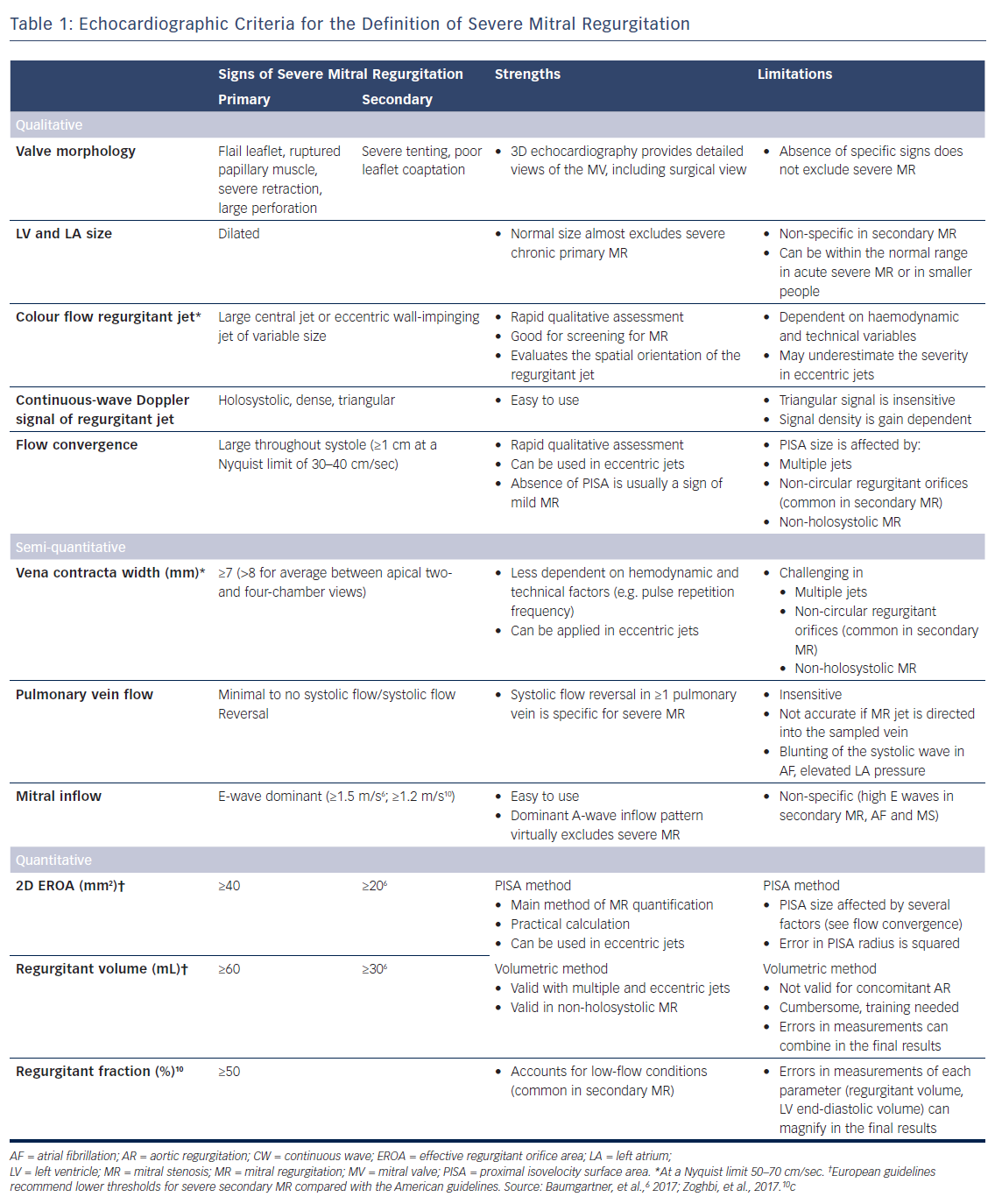
With the development of 3D echocardiography, the vena contracta area can be directly visualised using multiplanar reformation planes across the regurgitant orifice and measured by planimetry (Figure 1). Zeng et al15 proposed definition of severe MR to have a cut-off value of 3D vena contracta area as ≥0.41 cm2. In patients with functional MR, the 3D vena contracta area has been shown to be significantly larger than the 2D PISA-derived EROA (0.39 ± 0.17 cm2 versus 0.27 ± 0.11 cm2 respectively; p<0.001), resulting in an average 27 % underestimation of the EROA by the PISA method compared with the 3D vena contracta area.15
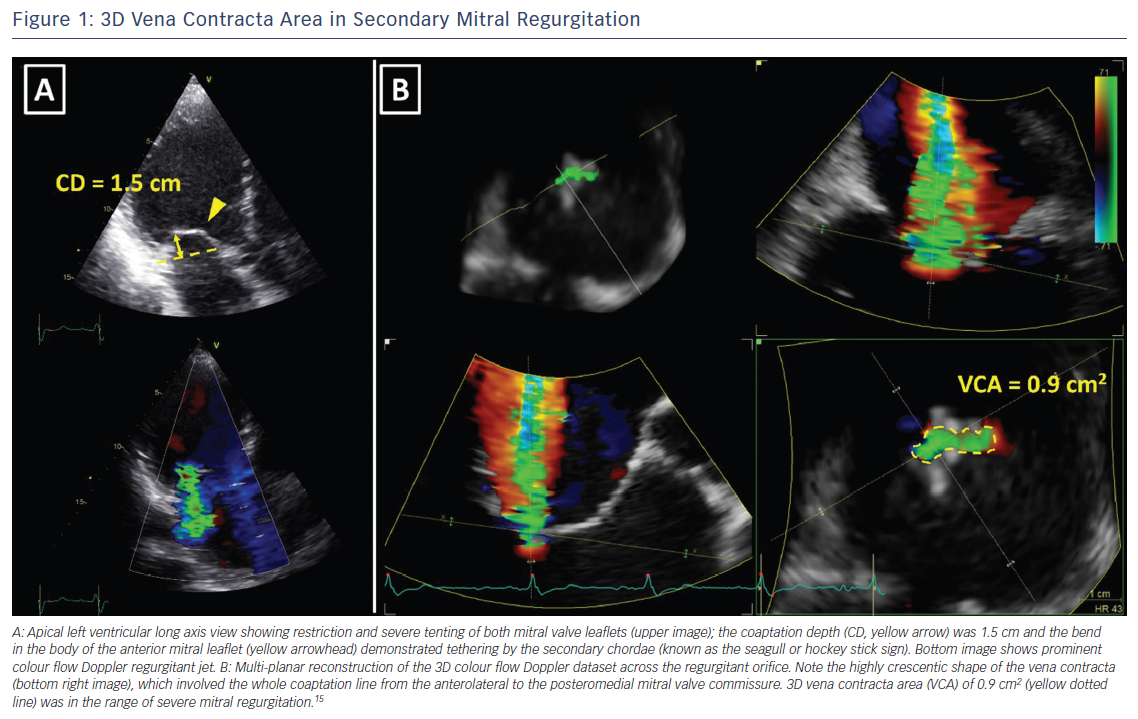
The assessment of the severity of MR with colour flow Doppler echocardiography is based on instantaneous peak flow rates and is therefore reliable only when there is little temporal variation of MR during the cardiac cycle. However, secondary MR is often dynamic, peaking in early and late systole and improving during mid systole when LV pressures are at their maximum.16 In such circumstances, MR should be quantified with volumetric methods, which account for the whole systole. In the absence of aortic regurgitation or intracardiac shunt, the difference between stroke volume measured at the mitral annulus (LV inflow) and the LV outflow tract (LV outflow) equals MR volume. Volumetric method is frequently used with CMR.6,7 The preferred method to quantify MR with CMR is to use phase contrast CMR to subtract the aortic forward flow from the LV stroke volume, assessed by planimetry of the LV short-axis cine images (Figure 2).10
Selecting Interventions for Mitral Regurgitation
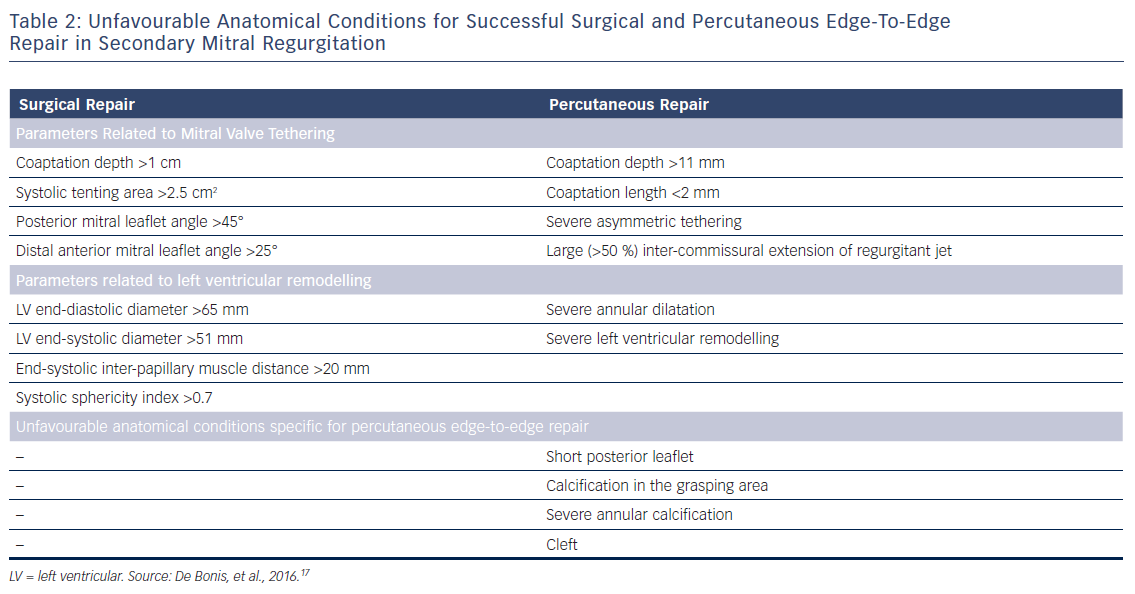
After establishing the diagnosis of symptomatic severe secondary MR, the type of valve intervention is based upon the degree of LV functional impairment, evidence of myocardial viability and the ability to perform revascularisation. When revascularisation is indicated, surgical intervention should be considered.6,8 However, the preferred type of surgical treatment, i.e. mitral valve repair by means of restrictive annuloplasty or chordal-sparing valve replacement, is not agreed upon. European guidelines recommend mitral valve repair as the preferred method, while mitral valve replacement may be considered in patients with echocardiographic risk factors for residual or recurrent MR (Table 2).6,17 In contrast, American guidelines recommend chordal-sparing mitral valve replacement for severely symptomatic patients (New York Heart Association class III–IV) with chronic severe ischaemic MR.8 This recommendation is based on the results of a randomised control trial that showed a higher rate of moderate or severe MR recurrence at 2 years follow-up in patients who underwent mitral valve repair compared with patients who underwent chordal-sparing mitral valve replacement (58.8 % versus 3.8 %, p<0.001), leading to higher incidence of HF and repeat hospitalisations in the mitral valve repair group.18 When revascularisation is not indicated, the decision between surgery and percutaneous edge-to-edge repair is made based on the degree of LV dysfunction and the surgical risk. When the surgical risk is low and LVEF is more than 30 %, surgery may be considered, while percutaneous edge-to-edge repair is preferred for patients presenting with high surgical risk or LVEF lower than 30 % despite optimal medical management, including pharmacological treatment and cardiac resynchronisation therapy.6 In the US, percutaneous edge-to-edge repair is currently not approved for clinical use in secondary MR.8
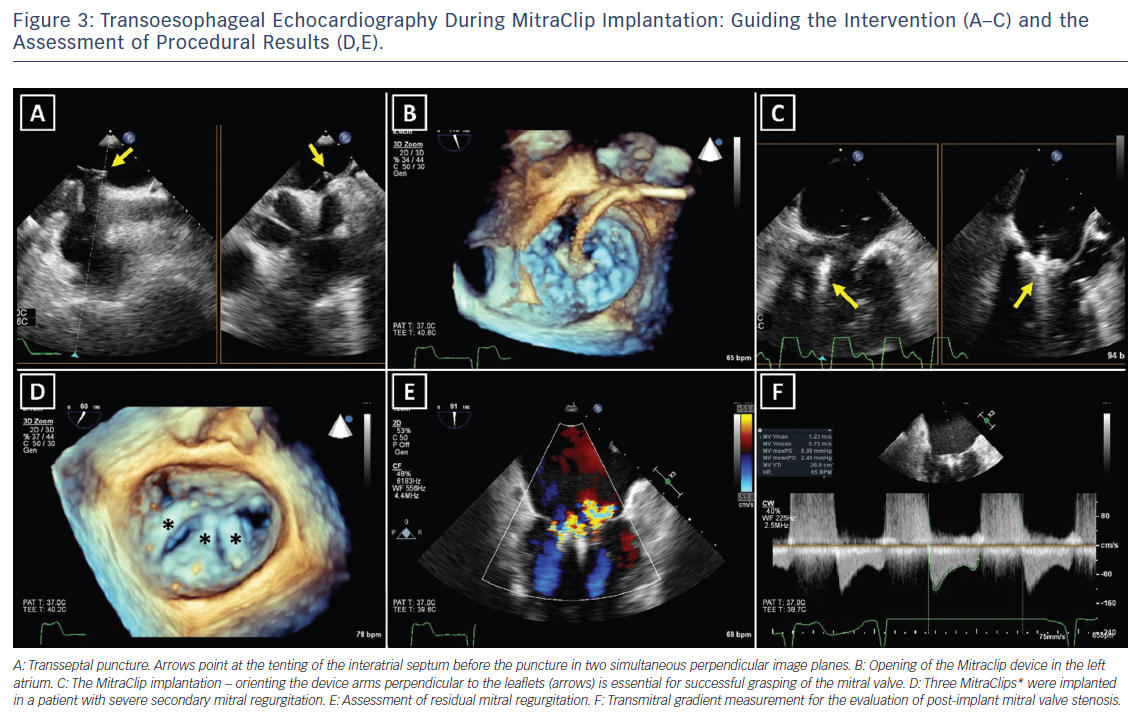
For successful surgical and percutaneous mitral valve repair in secondary MR, accurate LV assessment, including LV volumes, LVEF and sphericity index, is mandatory, accompanied by geometric assessment of the MV apparatus (tenting area, coaptation depth, leaflet angles and inter-papillary muscle distance). Transthoracic and transoesophageal echocardiography are the primary modalities, although detailed information can also be obtained with cardiac CT and CMR. Table 2 summarises the echocardiographic criteria that suggest increased risk of MR recurrence after mitral valve repair as well as unfavourable anatomical conditions for percutaneous edge-to-edge repair with a MitraClip® device (Abbott Vascular, Menlo Park, CA, US).17 In patients with secondary MR who are undergoing surgery, successful repair is less likely in the presence of severe mitral valve tethering with coaptation depth >1 cm, systolic tenting area >2.5 cm2, posterior mitral leaflet angle >45° and distal anterior mitral leaflet angle >25°.17,19 Furthermore, global and regional LV remodelling, indicated by LV end-diastolic dimension >65 mm, end-systolic dimension >51 mm, systolic sphericity index >0.7 and interpapillary muscle distance >20 mm predict a lower likelihood of successful mitral valve repair.17,20 A leaflet coaptation depth >11 mm and coaptation length <2 mm challenge the percutaneous edge-to-edge mitral valve repair since these parameters indicate advanced LV remodelling with excessive tethering of the mitral leaflets.17 Large regurgitant orifices often require implantation of more than one MitraClip to reduce MR. Short posterior leaflet, cleft, severe annular calcification and calcification in the grasping area are other anatomical conditions that challenge percutaneous edge-to-edge repair.17 Peri-procedural transoesophageal echocardiography is crucial to perform successful percutaneous implantation of a MitraClip device (Figure 3).
Aortic Stenosis in Heart Failure: Diagnosis and Assessment of Severity
The LV pressure overload caused by AS increases LV wall stress and as a consequence the LV responds with myocyte hypertrophy to maintain a normal LVEF. However, this response is counterproductive in the long term and causes LV diastolic dysfunction, myocardial ischaemia in the subendocardium, increased myocardial fibrosis (reactive and replacement) and eventually LV systolic dysfunction.21 Clinically, patients with severe AS may present with dyspnoea, chest pain and syncope.
The prevalence of HF among patients with severe AS varies largely based on the definition of HF (e.g. reduced LVEF and presence of symptoms) and the characteristics of people included in the studies. In one large cohort study (n=79,043) involving people with HF symptoms referred for echocardiography, mild-to-severe AS was found in 10.1 % and moderate or severe AS in 3.2 %.4 Furthermore, in the Euro Heart Survey, 19.3 % of people with severe AS undergoing surgical aortic valve replacement (SAVR) had LVEF <50 %.5 In a more contemporary study of 42,776 patients with AS undergoing SAVR included in the German Aortic Valve Registry, LVEF <50 % was present in 26.6 % of the patients.22 Data from the American Transcatheter Valve Therapy (TVT) Registry showed a 25.6 % prevalence of reduced LVEF (<45 %) among 42,988 patients undergoing TAVR.23
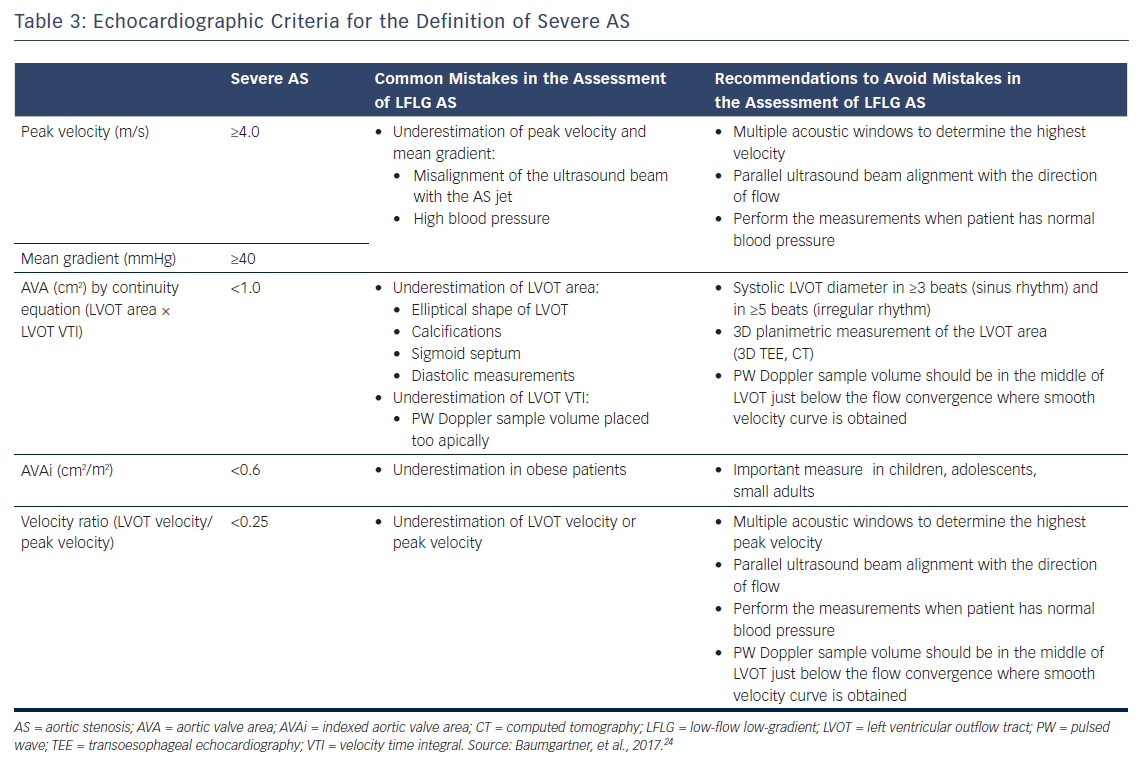
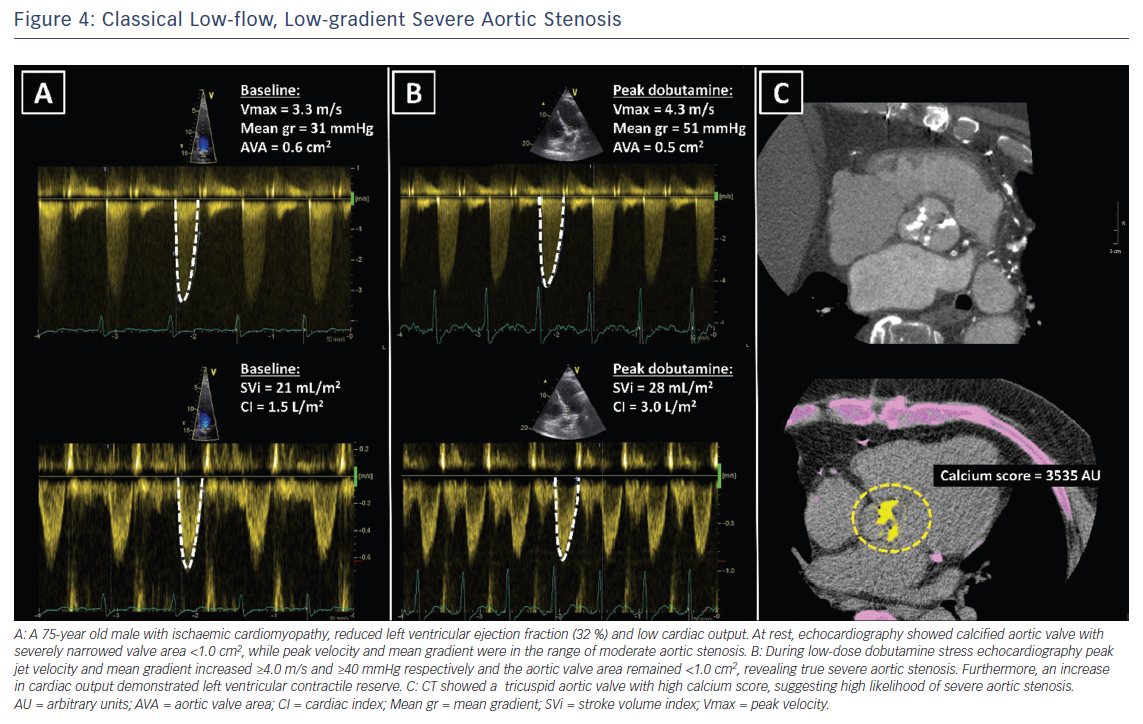
Doppler echocardiography is the preferred technique for the assessment of the severity of AS. The primary hemodynamic parameters defining severe AS with echocardiography are the peak jet velocity ≥4 m/s, mean transvalvular pressure gradient ≥40 mmHg and aortic valve area (AVA) by continuity equation <1 cm2 (Table 3).24 In the majority of patients, these criteria coincide. However, up to 30 % of patients may show low peak jet velocity and transaortic valve gradient with an AVA <1 cm2.25 This is frequently observed among patients with LVEF <50 %, the so-called classical low-flow, low-gradient severe AS.
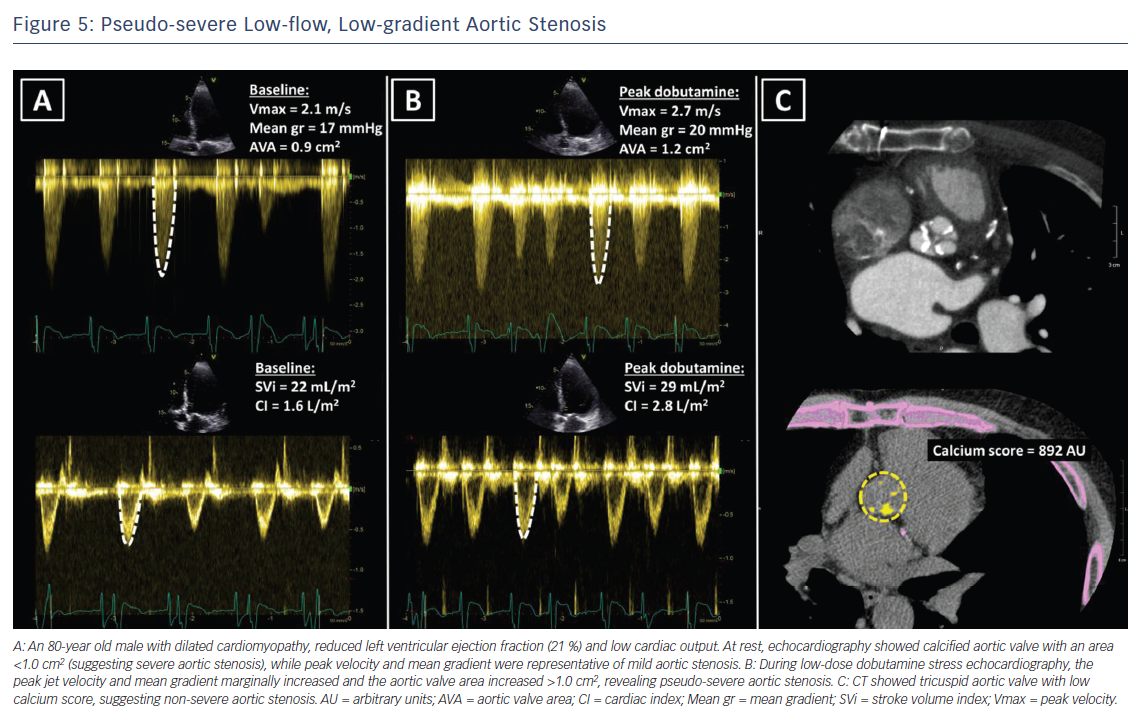
Low-dose dobutamine stress echocardiography is the primary diagnostic method to differentiate between true severe AS and pseudo-severe AS in patients with reduced LVEF.24 In patients with true severe AS, an IV infusion of low-dose dobutamine will increase the LV contractility and stroke volume leading to an increase in mean transvalvular gradient while the AVA will remain narrow (Figure 4). In contrast, pseudo-severe AS is diagnosed when the increase in LV contractility and stroke volume is accompanied by an increase in AVA of more than 1 cm2 (Figure 5). While patients with true severe low-flow, low-gradient AS should undergo prompt aortic valve intervention, the course of action for patients with pseudo-severe AS is less clear. Fougeres et al.26 demonstrated comparable survival of patients with pseudo-severe AS to that of propensity-matched patients with systolic HF and no evidence of VHD. However, this has recently been challenged by another study that demonstrated a very high risk for clinical events (defined as the composite of all-cause death, aortic valve replacement and HF hospitalization) among patients with HF and moderate AS.27 Furthermore, in a retrospective analysis of 1,090 people with moderate AS and LVEF ≤50 %, aortic valve surgery was associated with a higher 5-year survival compared with people who had medical therapy.28 While current guidelines do not recommend aortic valve intervention in HF patients with moderate AS, this view might change after the results of the ongoing international, multicentre, randomised trial TAVR UNLOAD, which has been designed to compare the efficacy and safety of transfemoral TAVR in addition to optimal HF therapy vs HF therapy alone in HF patients with moderate AS.29
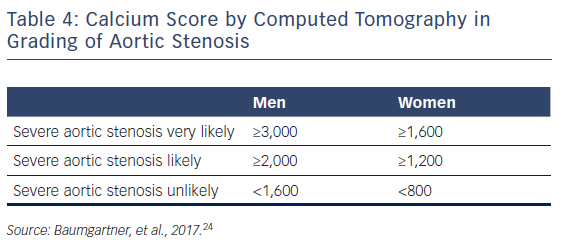
In patients without contractile reserve, defined as failure to increase stroke volume >20 % during dobutamine stress echocardiography, the assessment of aortic valve calcification burden with cardiac CT may help to estimate the severity of AS (Figures 4, 5).24 Aortic valve calcium score is quantified using the Agatston method and expressed in arbitrary units (AU).30 Cueff et al.31 demonstrated a good overall correlation between the degree of aortic valve calcification and hemodynamic parameters of AS severity assessed by: the AVA (r=-0.63, p<0.001); indexed AVA (r=-0.67, p<0.001); mean gradient (r=0.78, p<0.001); and peak velocity (r=0.79, p<0.001). The proposed cut-off value of 1,651 AU yielded a 93 % sensitivity and 75 % specificity in grading AS severity in patients with classical low-flow, low-gradient AS. Clavel et al.32 proposed different cut-off values to define severe AS for men and women as 2,065 AU and 1,274 AU, respectively. The joint European and American recommendations for the assessment of AS consider the aortic valve calcium score as a continuum – a very high calcium score suggests severe AS and a low calcium score suggests severe AS is unlikely (Table 4).24
Treatment Options for Aortic Stenosis
Current therapeutic options for patients with severe AS and HF are conservative medical therapy, SAVR and TAVR. The Europe and US Class 1 recommendation for patients with symptomatic high-gradient severe AS is that there is no lower LVEF limit for aortic valve intervention since LV function is likely to improve after relief of stenosis.6,7 The Class 1 recommendation for symptomatic severe AS patients with an LVEF <50 % is that they should undergo SAVR.6,7 In patients with classical low-flow, low-gradient severe AS with reduced LVEF, aortic valve intervention is indicated when dobutamine stress echocardiography shows evidence of LV contractile reserve. This is the Class 1 recommendation in European guidelines and Class 2a in American guidelines.6,7 An intervention should also be considered in patients without LV contractile reserve, particularly when the CT calcium score is high (Class IIa recommendation in European guidelines, while American guidelines stress the importance of individualised decisions in these high-risk patients).7,8 Tribouilloy et al.33 demonstrated that patients with low-flow, low-gradient severe AS without contractile reserve experience high operative mortality, but SAVR was associated with better outcomes compared with patients who were treated conservatively. Only symptomatic patients with severe comorbidities, in whom aortic valve intervention is unlikely to improve survival or quality of life, should be treated with medical therapy.6
The choice of the intervention in patients with symptomatic severe AS and HF should be made by the specialist heart team and should take into account the patient’s cardiac and extracardiac characteristics, the individual risk of surgery, the feasibility of TAVR, as well as the local experience and outcome data.6,8 Table 5 lists the imaging-derived characteristics that guide the decision to choose TAVR or SAVR. Multi-slice CT has become the imaging modality of choice for pre-procedural evaluation of TAVR candidates in most centres due to its low invasiveness and comprehensive evaluation.6 It allows assessment of the size and the shape of the aortic annulus, its distance to the coronary ostia, the distribution of calcifications and the dimensions of the aortic root, which is of paramount importance to determine feasibility of TAVR and to choose appropriate prosthesis size (Figure 6). However, if CT is contraindicated, for example, if the patient has severely impaired renal function, 3D transoesophageal echocardiography can be used to determine the aortic annulus size. It is important to remember that the obtained annulus dimensions with 3D transoesophageal echocardiography are smaller than those measured with cardiac CT and the echocardiographic accuracy can be reduced in heavily calcified aortic valves.34,35
Cardiac CT also allows assessment of the peripheral arteries to determine feasibility of transfemoral access, which is the least invasive TAVR approach, used in the majority of patients.23,36
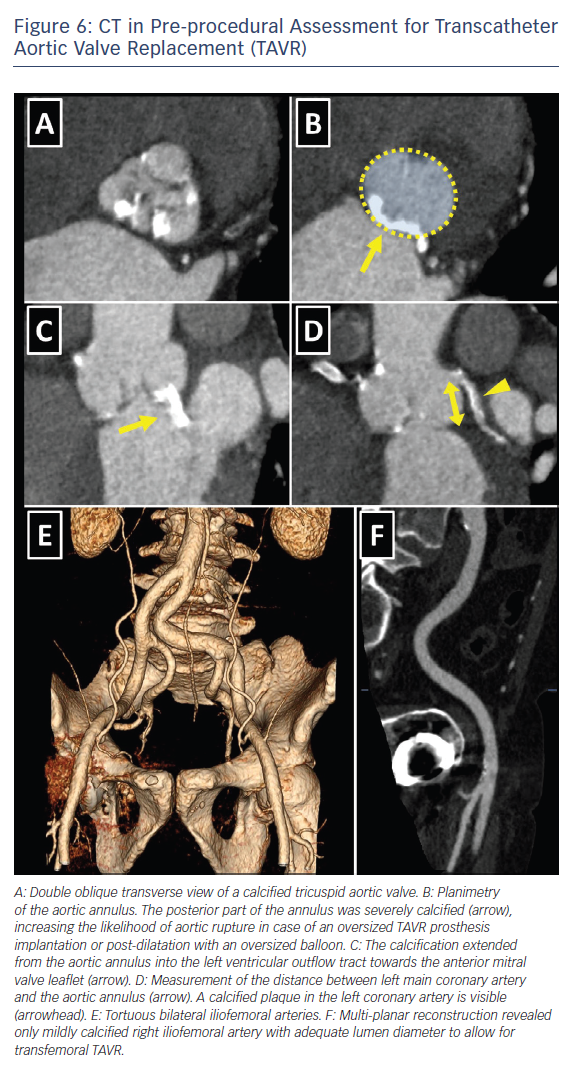
Cardiac CT allows detailed visualisation of iliofemoral arteries and aorta with the assessment of size, tortuosity, degree of calcification and plaque burden (Figure 6). For currently available TAVR delivery catheters, a 6–6.5 mm minimal luminal vessel diameter of femoral arteries is considered acceptable.37 In case of contraindications to CT, invasive angiography or, less commonly, CMR angiography might be employed.
Conclusion
Accurate grading of valvular lesion and reliable assessment of LV dysfunction is of paramount importance when deciding the most appropriate therapy for patients with VHD and HF. Transthoracic echocardiography is the first-line imaging modality to quantify LV systolic function and grade of valvular stenosis and regurgitation, as well as characterising the mechanism of valvular dysfunction. However, in HF patients, quantification of valvular dysfunction remains challenging and the use of other imaging techniques such as 3D transesophageal echocardiography, CMR and CT is needed to determine whether valve stenosis and regurgitation are severe. The integration of multimodality cardiovascular imaging is even more important when assessing suitability for transcatheter valve repair and replacement therapies. CT has become the key imaging modality for pre-procedural evaluation of patients undergoing TAVR, and 3D transoesophageal echocardiography is crucial to guide percutaneous edge-to-edge mitral valve repair.