Shock in cardio-surgical intensive care unit (ICU) patients is a serious condition with a high morbidity and mortality.1,2 Prompt identification of the underlying conditions and correct management of the life- threatening physiological deteriorations are crucial to save the patient’s life. In daily practice, however, focusing on the main goal – to provide adequate oxygen delivery thereby preventing further organ damage – is often difficult due to multiple influences, distractions, and pending results. Hence, the setting up of priorities is necessary. In addition, many questions arise on how to optimally treat patients in shock: What blood pressure should we target? What fluid should we use and how much? Which vasoactive drug is best and which inotrope? While discussions about these issues can be very fruitful for experts, they risk confusing younger colleagues and nurses at the bedside. Disputes about controversial therapy opinions might consequently delay adequate resuscitation, putting the patient at risk of an adverse outcome. This review aims to give recommendations for the management during the early phase of shock of cardio-surgical patients hospitalised in the ICU. The focus is on the first 6 hours, which are also called ‘the golden hours’.
Methods
Clinical Setting
The cardio-surgical ICU at the University Hospital Zurich is a 12-bed, high-intensity unit that cares for more than 1,100 patients every year. The majority of these critically ill patients undergo cardiac and/or major vascular surgery, most of them being mechanically ventilated and on inotrope/vasopressor support. The unit is part of a successful heart failure programme that includes extracorporeal life support, ventricular assist devices and a heart transplantation service. In order to optimise the initial resuscitation of patients and to avoid adverse effects of inadequate treatment, the consultants of the unit have agreed on treatment recommendations, which are presented here. They are based on current evidence and complemented by clinical experience in areas of uncertainty.
Data Extraction
For this narrative review, a search of the PubMed database and a review of bibliographies from selected articles were performed to identify original data relating to this topic. Key words used for the search were ‘shock’, ‘cardiac surgery’, ‘critical care’, ‘inotropes’, ‘inotropic therapy’, ‘circulatory support’, ‘cardiogenic shock’ and ‘sepsis’. National and international guidelines were reviewed and integrated, e.g. The 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation,3 The 2014 ESC/ EACTS guidelines on myocardial revascularization4 and The Surviving Sepsis Campaign guidelines5. Articles were scrutinised regarding their study design, population evaluated, interventions, outcomes, and limitations. Finally, personal recommendations were included and highlighted as such to give a comprehensive overview on this topic.
Rationale for an Early Management of Shock
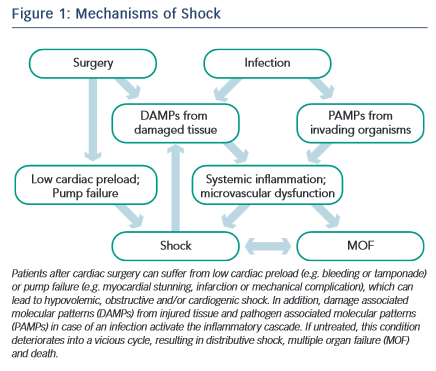
Shock in patients undergoing cardiac surgery occurs frequently with multiple potential causes at play.1,2Figure 1 depicts the interactions between surgery, infection, the systemic inflammatory response syndrome (SIRS), shock and multiple organ failure. The time between shock onset and shock resolution is one factor that defines the degree of organ dysfunction and the risk of death, as prolonged shock causes inflammation and irreversible tissue damage.6 Hence, prompt identification of patients with shock and immediate treatment are crucial for the outcome of such patients. The concept of goal-oriented resuscitation was pioneered by Shoemaker et al in high-risk surgical patients.7 Rivers et al published in 2001 that early goal-directed therapy (EGDT) significantly reduced mortality in patients with septic shock.8 Kumar et al demonstrated that early administration of appropriate antibiotics in septic patients with hypotension saved lives in a time- dependent manner.9 Accordingly, guidelines were published for the initial management of patients with sepsis and septic shock.5 Several studies have demonstrated a survival benefit for patients if such guidelines were implemented in clinical practice.10–12 The idea of EGDT was expanded to the clinical scenarios of acute heart failure13 and heart failure after cardiac surgery.14
More than 10 years later three large multi-centre trials re-evaluated the results of River’s landmark study: ARISE, ProCESS and ProMise.15–17 In these trials EGDT was not superior to standard care in patients with early septic shock.18 This might be explained by the fact that the included patients were less ill than in the original study. Second, the standard care groups might have benefited from early recognition and treatment of septic shock that have become the standard of care over the past decade. Third, continuous measurement of central venous saturation is not necessary to guide treatment of pathophysiological deteriorations. However, when shock does not resolve promptly with standard therapy, echocardiography and invasive haemodynamic monitoring become necessary to unravel the complex underlying mechanisms and to decide on further treatment. These principles can be implemented for all forms of shock, as the underlying pathophysiologic mechanisms (tissue damage, release of damage-associated molecules, inflammation, organ dysfunction) are similar in different shock states.
Definition of Shock
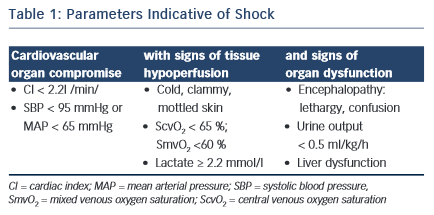
Shock is defined by the presence of global tissue hypoperfusion and signs of organ dysfunction resulting from severe cardiovascular compromise. Common clinical, haemodynamic and laboratory parameters indicative of shock are displayed in Table 1 (adapted from Kumar and Parillo2).
Four particular shock types can be distinguished (adapted from Kumar and Parillo2):
- Cardiogenic shock: Circulatory collapse due to pump failure of the heart (e.g. myocardial infarction, fulminant myocarditis)
- Hypovolemic shock: Inadequate cardiac preload due to fluid losses (e.g. postoperative haemorrhage, gastrointestinal bleeding, hypovolemia due to excessive use of diuretics)
- Distributive shock: Inadequate cardiac preload due to vasodilatation and vascular leakage (e.g. postoperative SIRS, sepsis, anaphylactic reaction)
- Obstructive shock: Inadequate cardiac preload due to obstructed venous return (e.g. pericardial tamponade, tension pneumothorax, abdominal compartment) or obstruction of arterial blood flow (e.g. pulmonary embolism).
Diagnostic Assessment
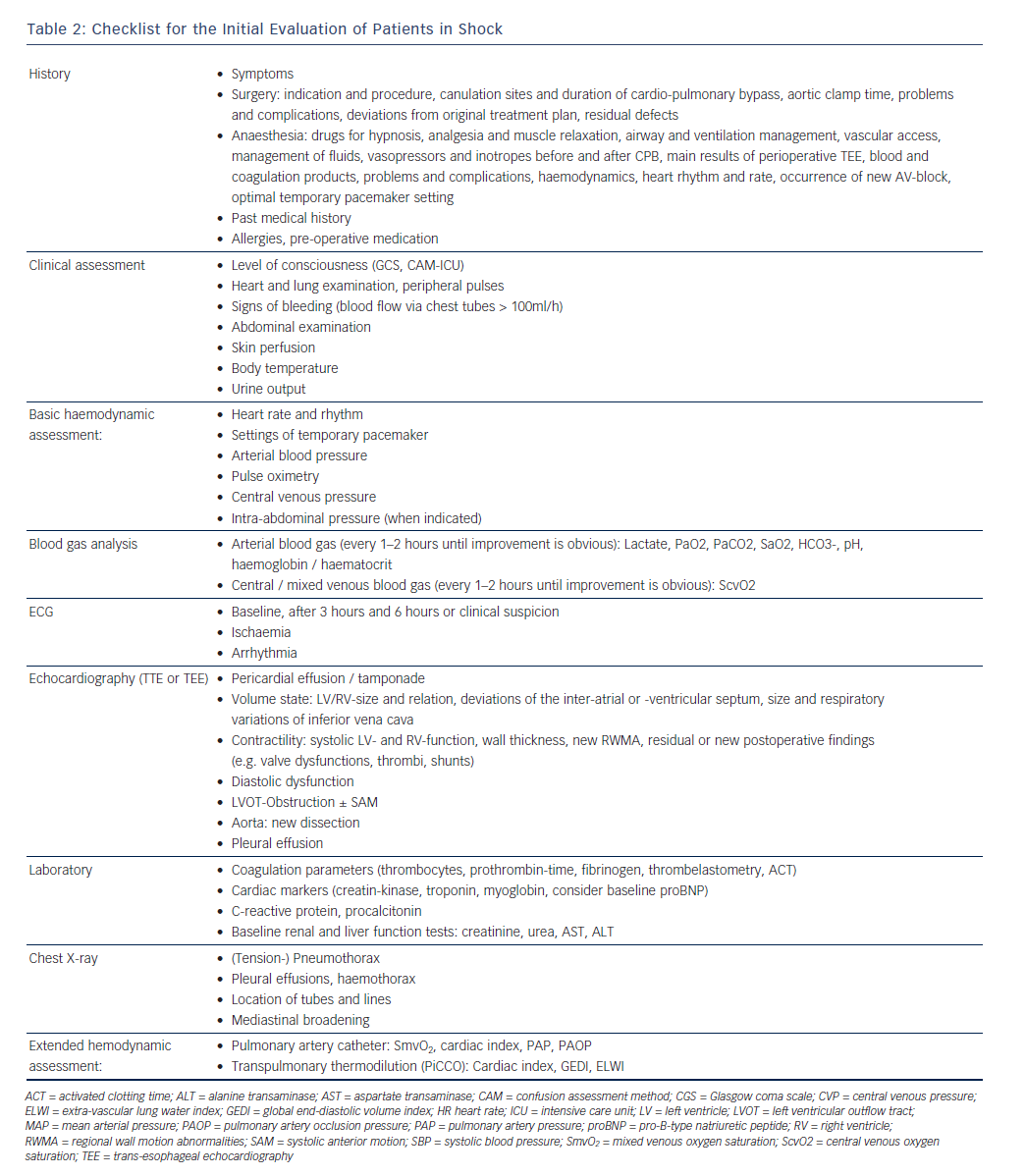
Table 2 describes a structured approach to the patient in shock. Physicians must be aware that simplifying the diagnostic process (i.e. cutting corners) bears the risk of false interpretation, particularly as patients after cardiac surgery often have more than one possible cause of shock. A thorough understanding of the obtained results in the clinical context is more important than the performance of the test itself. All values have to be interpreted and immediately implemented in the therapeutic decision process.
Echocardiography
A useful tool for the haemodynamic evaluation of patients with shock is echocardiography. It is readily available, non-invasive and can be performed after a reasonable period of training.19–21 However, expert advice should be available around the clock, as examination conditions in cardiovascular patients can be challenging (e.g. substernal air, dressings, chest tubes) and the underlying pathologies difficult to detect (e.g. localised pericardial effusion with tamponade, intracardiac shunts, dysfunction of artificial valves). If the quality of transthoracic imaging is insufficient, a transoesophageal echocardiography is indicated. Due to its semi-invasiveness complications may occur and contraindications should be respected. Oesophageal injury occurred in a single-centre study of 10,000 patients in fewer than one per 1,000 cases.21,22
Lactate
In hypoxic cells, glucose is metabolised to lactate, resulting in a much lower ATP yield as when glucose can be metabolised via pyruvate in the Krebs cycle under aerobic conditions. Therefore, lactate is a useful marker of an energy crisis at the cellular levels in patients with shock. Whereas in low-flow shock states a lactate elevation is often caused by tissue hypoxia, in distributive shock the mechanisms are more complex and involve the increase in glycolysis, inhibition of pyruvate dehydrogenase23 and the stimulation of muscular Na+/ K+ ATPase.24 High lactate plasma levels and insufficient lactate elimination are associated with poor outcome.25–27 Consequently, lactate has proved to be a useful parameter for evaluation of the resuscitation process of patients with septic shock.28 In the study by Jones et al, packed red blood cells were transfused if lactate clearance was <10 % within 1 hour and haematocrit was <30 %; dobutamine was initiated if lactated clearance remained low.29 Other investigators demonstrated a mortality-reduction in patients with a blood lactate level > 3mmol/l, when a decrease of 20 % in the blood lactate level over 2 hours was targeted.30 Nevertheless, because of its complex metabolism and physiology, elevated lactate levels per se are not an indication for the administration of fluids or inotropes; lactate values might change more slowly than other haemodynamic parameters of perfusion.
Importantly, lactate is also released by fully oxygenated tissues.31,32 The production is stimulated by adrenaline and lactate, in this case rather fuel than waste product, acts as an energy shuttle from skeletal muscles to vital organs, such as the heart and brain.33,34 In addition, lactate elevations may result not only from increased production but also from decreased elimination as in acute liver dysfunction. In conclusion, elevated lactate levels must be interpreted with caution as they do not in every case indicate tissue hypoxia.34,35
Treatment Recommendations
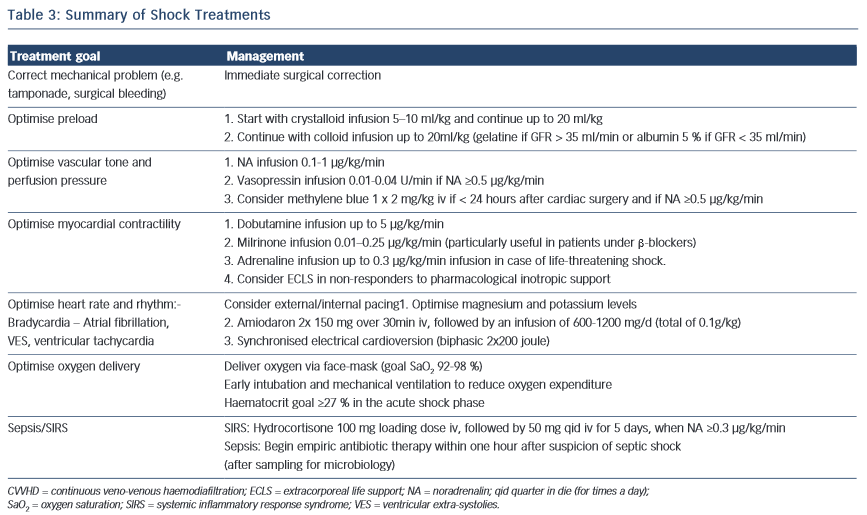
Once shock is identified, the most important goal is the correct diagnosis of the underlying cause and the mechanisms at play. Simultaneously, prompt resuscitation measures are undertaken to provide adequate oxygen delivery and to prevent further organ damage. Tissue oxygenation is adequate when the balance between oxygen consumption and delivery is met. Treatment recommendations are summarised in Table 3. While there are clear differences in the treatment of cardiogenic shock and septic shock patients (e.g. revascularisation versus source control), there are also similarities (e.g. vasopressors in cases of decreased vascular tone).
Optimise preload
Fluid therapy is recommended to re-establish vascular filling and cardiac preload in patients with shock (Tables 3 and 4). However, despite considerable research efforts resulting in more than 4,000 articles (including more than 860 reviews) listed in PubMed.gov, the best fluid for shock resuscitation is still a matter of debate. Adverse effects and costs vary according to the fluids used, without clear benefit favouring one solution over others. A large observational study revealed, that gelatin 4 % solutions and hydroxyethyl starch (HES) 6 % (130/04) led to a similar dose-related increase of renal failure in critically ill patients.36 In contrast, fluid resuscitation with only crystalloids significantly reduced the incidence of acute kidney injury in severe sepsis patients.37 The 6S study showed increased mortality and need for renal replacement therapy in the HES group compared with the use of crystalloids.38 There is evidence that HES preparations are stored in human tissue and are associated with kidney failure,39,40 increased bleeding risk41 and higher mortality.39,42 Therefore, and because of the findings above, HES is no longer used in the Zurich ICU.
Large volume transfusion with 0.9 % saline may result in hyperchloremic metabolic acidosis.43 Additionally, studies show that the use of balanced salt solution, compared with 0.9 % saline, are associated with a decreased rate of infections, renal dysfunction, and blood transfusion.44–46
The SAFE study, a large multi-centre study comparing normal saline with albumin 4 % solutions, found a similar mortality with both solutions irrespective of baseline albumin concentrations.47,48 Post hoc analysis revealed a decreased mortality risk in the subgroup of septic patients49 when treated with albumin 4 %. In another study, albumin was associated with a lower mortality compared with other fluid regimens.50 In a retrospective study with almost 20,000 patients undergoing coronary artery bypass surgery, Sedrakyan et al compared the effect of different colloids on the postoperative mortality.51 Albumin was used in more than 8,000 patients and non-protein colloids (HES and dextran) in the rest of the cohort. The mortality rate was 25 % lower in patients receiving albumin. This difference might be explained by a better maintenance of vascular integrity during ischaemia-reperfusion,52 the reversal of hypoalbuminaemia (a strong risk factor for mortality),53 as well as by excessive bleeding associated with starches. Gelatin and HES reduced maximum clot firmness of thromboelastometry tracings, whereas these values remained unchanged after administration of albumin.54 The major disadvantages of current available human albumin solutions include high cost, its delivery via glass bottles and the potential, although small, risk of viral infections. Taking these partly conflicting results into account, a fluid regimen as described in Table 3 is suggested. Details of the proposed fluids are summarised in Table 4.
Maintain Perfusion Pressure
Providing an adequate perfusion pressure during shock by restoring (or increasing) blood pressure is a key intervention in the treatment of shock. Most reviews and guidelines recommend a target MAP of 65 mmHg.23,55
When hypotension persists despite a fluid challenge, vasopressors are started. Noradrenalin is used because of its -adrenergic and -adrenergic properties. stimulation increases vascular tone while stimulation improves contractility. Noradrenalin is the vasopressor of choice in septic shock5 as well as in other forms of shock. A limiting factor is excessive vasoconstriction and organ ischaemia, which is sometimes difficult to distinguish from ischaemia caused by inadequate resuscitation.56 Adrenalin can be used in acute life- threatening shock. At low doses, mainly -adrenergic effects are transmitted, while -adrenergic effects dominate at high doses. Important side effects of adrenalin are arrhythmias, metabolic effects (hyperglycaemia, hyperlactaemia) and a decrease in splanchnic blood flow, bearing the risk of mesenterial hypoperfusion.57,58
Vasopressin is used in patients with noradrenalin doses >0.5 mcg/kg/min. In septic shock, vasopressor deficiency can occur59 and its supplementation is safe60–62 with a catecholamine sparing effect.63 A survival benefit could not be shown for patients with mild septic shock and for those who received glucocorticoids.23,62,64 Patients with cardiogenic shock might even be harmed by the increase in afterload and the coronary vasoconstriction.65 In accordance with others, a maximum dose of 0,04 U/min is recommended.5,23
Methylene blue prevents vasodilation by inhibiting soluble guanylyl cyclase and nitric oxide synthase activity. The optimal dose is unknown,66 as well as its effect on morbidity and mortality.67 Methylene blue (2 mg/kg iv) in persistent shock should only be used during the first 24 hours following cardio-pulmonary bypass. It is worth noting, that the Surviving Sepsis Campaign does not mention methylene blue in its recommendations.5
Control Heart Rate and Synchronise Heart Rhythm
A new AV-block or atrial fibrillation is common after cardiac surgery.68–70 Temporary epicardial pacemaker wires are routinely implanted during surgery for ventricular pacing in case of postoperative third degree AV-block. In cases of severe intraoperative bradycardia or asystole, temporary atrial pacing may be useful for better haemodynamic stability; an increase in heart rate increases cardiac output if AV synchrony is maintained.71 As a general rule, the more physiological stimulation applied (AAI>DDD>VVI), the better the result on cardiac output.
Atrial fibrillation impairs ventricular filling thereby potentially contributing to the shock state.72,73 In patients with haemodynamic instability, conversion into sinus rhythms must be attempted by direct current electrical cardioversion. Synchronised cardioversion with anterior-posterior electrode positioning and high initial biphasic energy of 200 joules is recommended.72,74 Prior to this, electrolytes are supplemented to obtain high plasma levels (magnesium >1.0 mmol/l; potassium 4.5–5.5 mmol/l).72,75,76
Improve Contractility
Inotropes are used in patients with low cardiac output due to impaired cardiac contractility.5 Details on recommended drugs and dosages are listed in Table 3.
Dobutamine has mainly 1-adrenergic properties and increases contractility with little effect on peripheral vascular 2-receptors causing some degree of vasodilatation. Although improving oxygen delivery was beneficial perioperatively4,75 and during the early phase of shock,8 circulatory stimulation with dobutamine to supra-normal values (cardiac index > 4.5 l/min/m2, oxygen delivery >600 ml/min/m2) was associated with an adverse outcome in patients with established multi-organ failure.78,79 This might be due to the fact that excessive adrenergic stimulation has adverse metabolic effects (augmented energy demands, hyperglycaemia, lipolysis, muscle wasting),80–82 impairs splanchnic haemodynamics,83,84 causes arrhythmia,85 stimulates inflammation86,87 and promotes stress cardiomyopathy.88,89 Dobutamine is also associated with eosinophilia and fever,90 and its use for longer than 72 hours may lead to tolerance.
Adrenaline has potent effects on inotropy and chronotropy via-adrenergic receptors and produces vasoconstriction via -adrenergic receptors.91 A dose of only 0.03 μg/kg/min adrenaline compared with placebo resulted in a significant increase of cardiac output and in a significant rise in MAP.92 In a small pilot study, adrenaline and the combination of dobutamine-noradrenaline improved both cardiac output and oxygen delivery in patients with cardiogenic shock but a transient lactic acidosis, an increase in insulin requirements, impairments in gastric mucosa perfusion, (supra-)ventricular arrhythmia and an increased myocardial oxygen consumption were observed in the adrenaline group.57 The adrenaline-induced increase in lactate levels may mislead to excessive fluid loading or other potentially harmful shock therapies.57
Milrinone is a phosphodiesterase type III inhibitor and augments the intracellular concentration of cAMP. In comparison to dobutamine, milrinone is considered to be more effective in patients under-blockade.80,90 The effects of milrinone on right ventricular ejection fraction were comparable with the inhalational nitric oxide at 20 ppm.93 However, systemic vasodilation limits the use of milrinone in shock. A loading dose (50 mcg/kg) might profoundly worsen hypotension and is not recommended in ICU patients. The half-life of 50 minutes is longer in comparison with dobutamine or adrenaline and hypotensive effects will be prolonged if renal function is impaired.90
In summary, -mimetic inotropes increase contractility but can have serious adverse effects. Hence, their use should be limited to the lowest dose and the shortest duration necessary. The use of Levosimendan will be discussed in a planned future article for Cardiac Failure Review.
Increase Oxygen Transport Capacity
In a recent multicentre trial 2,007 patients undergoing non-emergency cardiac surgery were randomly assigned to a restrictive (haemoglobin <75 g/l) or a liberal transfusion threshold (haemoglobin <90 g/l). No differences concerning morbidity or health costs between the two groups were shown but deaths increased in the restrictive-threshold group (4.2 % vs 2.6 %, P=0.045).94 In patients with septic shock, no differences in outcome were shown between patients receiving blood transfusion at a threshold of 70 g/l compared to a threshold of 90 g/l.95 Strikingly, 50 % fewer units of blood were used in the low-threshold group. Importantly, blood transfusions have been associated with an increased morbidity94,96 and mortality97,98 after cardiac surgery. According to local policy in Zurich, a restrictive transfusion strategy is followed. After targeting a haematocrit of 27 % in the acute shock phase, a haematocrit level as low as 21 % is tolerated if no signs of oxygen misbalance (e.g. arrhythmias, ST segment changes, low SvO2, rising lactate levels, worsening metabolic acidosis) or obvious blood loss are evident. The indication for transfusion should be verified individually and for every single blood unit in the authors’ opinion.
Consider Extracorporeal Life Support
If shock does not resolve with pharmacological support, arterio- venous extracorporeal life support (ECLS) should be considered. ECLS can be used as a bridge to decision, bridge to bridge or bridge to transplantation.99 The possibility of rapid insertion of peripheral veno-arterial cannulas in shock is one of the advantages of ECLS.
This can even be done in conscious patients, thereby avoiding the risks of sedation, intubation and mechanical ventilation.100 Intra- aortic balloon pump (IABP) increases diastolic arterial pressure as well as coronary perfusion and decreases afterload, systolic blood pressure and myocardial oxygen consumption. However, no benefit on 30-day mortality could be demonstrated for the use of IABP in cardiogenic shock.101,102 In smaller trials, a benefit was found for IABP in patients with acute ischaemic mitral regurgitation and other mechanical complications of myocardial ischemia.103,104 Hence, IABP can only be recommended in selected patients with mechanical complications as bridge to cardiac surgery. The IABP is also inserted postoperatively in patients with decompensated left-sided heart failure of primarily ischaemic aetiology (with or without secondary mitral regurgitation) as bridge to recovery, when increasing doses of inotropes are necessary to stabilise the patient.”
Fight Bacterial Infections
Surgical infection control (e.g. sternal wound infection) and empiric antibiotic therapy are most important in the fight against bacterial infections. In septic shock, the early administration of intravenous antibiotics improves outcome, as mortality increased 7.6 % for each hour antibiotics were delayed after the onset of hypotension.9 According to the Surviving Sepsis Campaign guidelines, empiric antibiotics should be administered within the first hour after sepsis recognition.5,105 Cultures of blood and other specimens should be obtained before the first administration of antibiotics.5
Consider Anti-inflammatory Strategies
In patients after cardiac surgery, an inflammatory response is initiated by the surgical trauma itself (Figure 1), contact activation of the inflammatory cascade by the bypass circuit and ischaemia- reperfusion injury.106–109 Conflicting studies exist on the use of steroids after cardiac surgery, some of them reporting a decrease in catecholamine support and a shorter ICU stay.110,111 Supplementation of 50 mg hydrocortisone iv qid is a place to start when distributive shock persists and the noradrenaline requirement is > 0.3 mcg/kg/min iv.
Conclusions
In postoperative cardiac patients, shock is a serious condition with a high morbidity and mortality. Cardiogenic, hypovolemic, obstructive and distributive shock can occur alone or in combination. Early identification of the underlying diseases and understanding the mechanisms at play are key for the correct management of these patients. Prompt resuscitation measures are necessary to reverse the shock state and to avoid permanent organ dysfunction or death. Treatment includes surgical correction when indicated, preload optimisation, maintenance of perfusion pressure, heart rate and arrhythmia control and inotropic support. Although highly effective when used in the right patient at the right dose and the right time, all therapies have the potential for adverse effects. Hence, patient management should assess the benefits against the risks of the various therapeutic interventions.112 This review focuses on the first six hours – the ‘golden hours’. The next phase of ICU management– the ‘silver days’ – will be reviewed in a planned future article for Cardiac Failure Review.