Heart failure (HF) remains an important cause of morbidity and mortality worldwide. The prevalence of HF in the developed world approximates 1–2% of the adult population, rising to >10% in those aged >75 years.1 Three distinct phenotypes based on left ventricular ejection fraction (LVEF) have been described: HF with preserved ejection fraction (HFpEF), HF with mildly reduced ejection fraction and HF with reduced ejection fraction (HFrEF).2 Approximately half of all HF patients have reduced ejection fraction (LVEF <40%) at rest.3
What makes cardiac imaging the cornerstone of the assessment of HFrEF is the identification of impaired left ventricular contraction and reduced left ventricular ejection fraction, which are the essentials of diagnosing HFrEF, which is followed by initiating disease-modifying therapy. Furthermore, identification of the underlying aetiology is of great importance, allowing the personalisation of treatment and prognostication. In addition to providing accurate volumetric assessment, cardiac imaging has evolved to offer functional, haemodynamic and tissue characterisation. This review summarises the role of cardiac imaging in HfrEF, with a focus on diagnosis, phenotyping, assessment of aetiology, therapy planning and prognostication.
The Role of Imaging in the Diagnosis of HFrEF
The current European Society of Cardiology guidelines define HFrEF as the presence of signs and symptoms of HF and a reduced ejection fraction (LVEF ≤40%).2 Accordingly, accurate estimation of LVEF remains the cornerstone of HFrEF diagnosis. 2D transthoracic echocardiography (TTE) remains the initial modality recommended for the assessment of LVEF. 2D LVEF is typically calculated using the biplane method of disks (modified Simpson’s) method. Measurement areas are acquired in two single planes from which inferences are made to estimate the LV shape and obtain 3D volumes (Figure 1A). This method relies on assumptions regarding the geometric shape of the LV. Moreover, poor image quality can lead to foreshortening of the ventricles and, therefore, underestimation of the volumes. If required, intravenous contrast can be administered to better delineate the endocardial border, increasing the feasibility of biplane volume analysis and improving the accuracy of LVEF estimation.4 This may concomitantly allow the diagnosis of LV thrombus when present.
More comprehensive evaluation of LV systolic function is possible using advanced imaging techniques, such as 3D echocardiography, speckle tracking and cardiac MRI (CMR). 3D echocardiography provides volumes with minimal postprocessing requirements, and is more accurate and reproducible than 2D assessment. However, it remains dependent on good acoustic windows and scanning technique, rendering it subject to many of the same limitations as 2D TTE.
Assessment of myocardial deformation is an emerging technique that may have an important clinical role in the diagnosis of HFrEF. Speckle tracking echocardiography analyses LV deformation by tracking cardiac motion from image intensities (the speckled pattern of the myocardium). It allows fast and highly automated LV chamber quantification, producing accurate and reproducible LVEF estimation.5 Moreover, it permits the measurement of myocardial strain and strain rate. Strain is defined as the change in length of a myocardial segment relative to its resting length, while strain rate is defined as the rate of such deformation.6 Global longitudinal strain may be more sensitive than LVEF as a measure of LV systolic function, allowing early changes in patients at high risk to be detected.7,8 Moreover, it improves risk prediction beyond LVEF assessment in patients with HFrEF.9 It is calculated as the average of the peak systolic longitudinal strain from all LV segments in apical four, three and two chamber views. Twist, torsion and twisting rate are other indices of systolic function that can be measured on speckle tracking echocardiography.
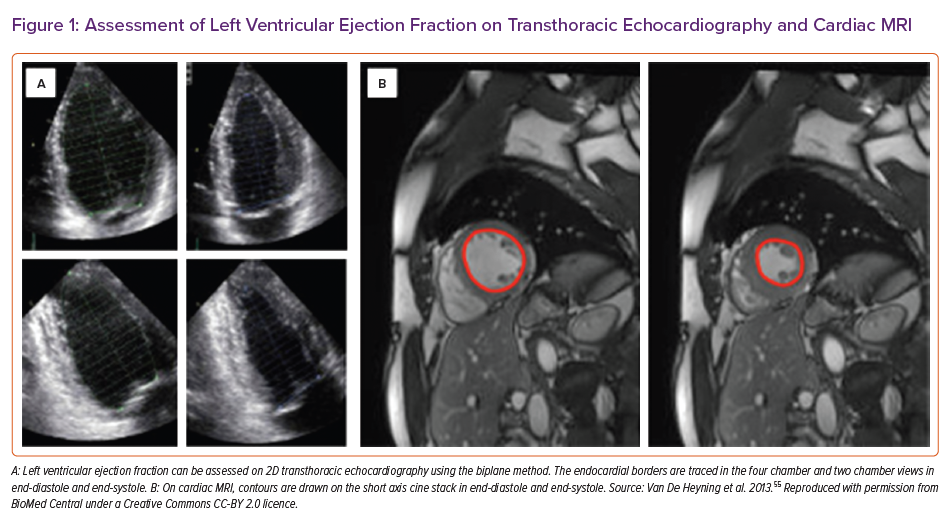
CMR has an important complimentary role in the early assessment of patients with HfrEF, and has emerged as the gold standard for the assessment of LV volumes and LVEF.10 Its use is recognised as a class 1 recommendation in the diagnosis of HF in patients with suboptimal TTE imaging.2 The superior resolution of CMR permits highly accurate and reproducible quantification of LV volumes, mass and LVEF (Figure 1B). Furthermore, steady-state free precession techniques provide good delineation of the blood-to-myocardium interface, allowing regional wall motion abnormalities to be easily identified. CMR is also particularly well suited to study the right ventricle (RV), which is often poorly imaged by TTE. Furthermore, CMR has the ability to go beyond LVEF assessment, thus with the use of tissue characterisation, phase contrast imaging and, in the right circumstances, stress perfusion imaging can provide a comprehensive assessment of HFrEF aetiology, physiology and function in one sitting.
LVEF can also be estimated with nuclear imaging techniques. This can be achieved using first-pass radionuclide ventriculography, equilibrium blood pool ventriculography or gated single-photon emission CT (SPECT). These techniques are rarely used as a first line due to high availability of TTE; however, they could provide an appropriate alternative where TTE is non-diagnostic, particularly where information on perfusion is simultaneously required, thus gated SPECT imaging could be used.11
One problem with the assessment of LVEF is that the results of measuring LVEF in the same patient using different imaging techniques can yield varied results.12,13 This is particularly important in those with marginal reduction of LVEF, which could lead to the diagnostic dilemma of inappropriate classification of HF. In addition, some interventions in HFrEF are linked to LVEF thresholds, yet the guidelines recommending these thresholds rarely touch upon the method of calculating the LVEF.
The Role of Imaging in the Assessment of Aetiology of HFrEF
HF is a complex clinical syndrome that can be secondary to a wide range of cardiac conditions, including hereditary defects and systemic diseases. The treatment of which, is in part, determined by the identification of the underlying disease process. As such, advanced cardiac imaging has a key role in determining HF aetiology.
Determination of HF aetiology begins with TTE assessment, which has the ability to identify significant valvular lesions, regional wall motion abnormalities, which, in the appropriate clinical context, may be indicative of ischaemic aetiology of HF, and quantification of increased wall thickness caused either by hypertrophy in response to pressure load or due to cardiomyopathy, or by infiltrative disorders. However, when a definitive diagnosis is not achieved with TTE, the most useful investigation is CMR with tissue characterisation. Tissue characterisation techniques include inversion recovery images acquired either early (for thrombus imaging) or late after contrast administration, diffuse fibrosis assessment with T1 mapping and extracellular volume measurement, oedema evaluation using T2-weighted images, and iron concentration using T2*.
The presence, distribution and extent of late gadolinium enhancement (LGE) provides valuable information on aetiology and can exclude ischaemic heart disease as a potentially reversible cause. Beyond imaging MI, LGE is an invaluable tool for identifying myocardial scarring in other cardiomyopathic processes. Compared with normal myocardium, the wash out of gadolinium in a myocardial scar is delayed, which results in a bright signal on inversion recovery images.
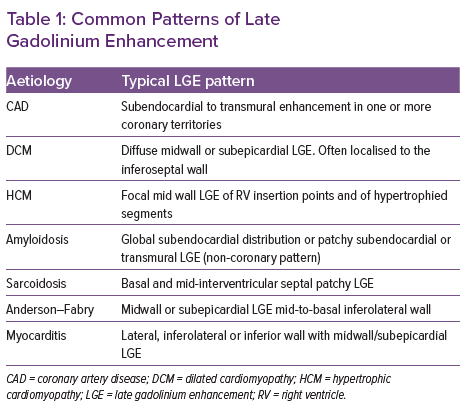
Different patterns of LGE have been described, primarily categorised into ischaemic and non-ischaemic patterns (Table 1). Ischaemic necrosis spreads from the subendocardium to the epicardium with increasing coronary occlusion time. Therefore, infarct-related LGE is typically subendocardial with increasing transmurality with increasing infarct severity, and is confined to one or more coronary territories.14 Conversely, midwall enhancement is typically seen in dilated cardiomyopathy and infiltrative disorders.
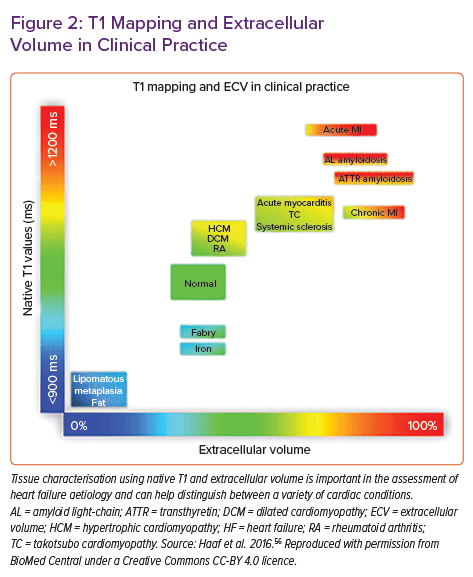
As well as assessing focal fibrosis with LGE, CMR can provide information on diffuse fibrosis with T1 mapping techniques. With LGE, diffuse fibrosis can go undetected because of the absence of normal reference myocardium. Furthermore, the presence of microscopic interstitial fibrosis is limited by the spatial resolution of LGE. T1 mapping involves the creation of a pixelated map obtained by measuring the longitudinal relaxation time of individual protons while they re-equilibrate following excitation with the radiofrequency beam. T1 increases in the presence of oedema (e.g. in acute infarction or inflammation) and with an increase in interstitial space (in the presence of fibrosis or scar and in amyloid deposition). Conversely, T1 is reduced by lipid overload (e.g. Anderson–Fabry disease and lipomatous metaplasia in chronic MI) and iron overload. Furthermore, there are growing data suggesting a correlation between T1 relaxation time and circumferential strain, as well as LV diastolic function.15 When acquired with contrast, the extracellular volume (ECV) fraction of the myocardium can also be calculated. Estimation of the ECV (interstitium and extracellular matrix) requires measurement of myocardial and blood T1 before and after administration of contrast agents, as well as the patient’s haematocrit value according to the formula:
ECV = (1-hct) [(1/pT1my – 1/nT1my) / (1/pT1bp – 1/nT1bp)]
Where nT1 is native T1, pT1 is postcontrast T1, my is myocardium, bp is blood pool and hct is haematocrit.
ECV is a marker of myocardial tissue remodelling and provides a physiologically intuitive unit of measurement. An increased ECV is most often due to excessive collagen deposition in areas of fibrosis (Figure 2). T2 imaging has a role in depicting oedema due to the effect of increased interstitial-free water on lengthening T2 relaxation times, this is particularly relevant in inflammatory conditions, such as myocarditis, sarcoidosis and acute ischaemic injury.
Finally, T2* imaging can be used to investigate suspected cardiac iron overload. The magnetic relaxation property of any tissue is inversely related to intracellular iron stores. A T2* of <20 is a reproducible and specific marker of cardiac iron content. Importantly, this often declines before LVEF, and is the best predictor of future HF and arrhythmia in these patients. If chelation therapy is started early, the reductions in T2* are reversible. Therefore, early identification is of great clinical importance. Diffusion tensor CMR is an emerging technique that can infer the microstructure of the myocardium by assessing the diffusion of water, and is a novel way of phenotyping HF at the cellular level. Aberrant sheetlet orientation has been demonstrated in both dilated cardiomyopathy and hypertrophic cardiomyopathy. This is largely a research tool at present, with some promise in the future.
Coronary artery disease is the most common cause of HFrEF in developed nations. CT coronary angiography (CTCA) and non-invasive functional imaging techniques have a role in identifying ischaemic aetiology in HF, and in determining reversibility of ischaemia and suitability for coronary intervention. CT coronary angiography provides high accuracy for the detection of obstructive stenoses, as defined by invasive coronary angiography, but is less equipped to determine functional lesion significance. Newer techniques, such as CT perfusion imaging and CT-derived fractional flow reserve, begin to address this, and improve the diagnostic accuracy of CTCA when compared with invasive fractional flow reserve.16 The identification of ischaemia can be achieved on non-invasive functional imaging tests, including stress nuclear imaging, stress echocardiography or stress perfusion CMR. Ischaemia can be provoked by exercise or pharmacological stressors, and is then identified through the presence of wall motion abnormalities on stress CMR or stress TTE, or perfusion changes on contrast enhanced echocardiography, SPECT, PET or contrast-enhanced perfusion CMR.
Fluorodeoxyglucose (FDG) PET imaging can also be used to identify active sarcoidosis in patients with suspected cardiac involvement.17 Moreover, a hybrid PET/CT approach has the advantage that it can visualise FDG accumulation in activated inflammatory cells and simultaneously provide whole-body PET and CT images.
Nuclear techniques have a role in the diagnosis of cardiac amyloidosis.18 Scintigraphy with technetium labelled bisphosphonates localise to transthyretin cardiac amyloid deposits, and these techniques demonstrate high sensitivity and specificity in detecting cardiac amyloid. The use of 123I-metaiodobenzylguanidine (MIBG) has been examined in the diagnosis of cardiac amyloid. MIBG uptake is significantly reduced in the presence of amyloid deposits, especially in familial amyloid polyneuropathy, which is characterised by early autonomic system involvement. MIBG imaging in this context may allow early detection of amyloid before TTE features emerge.19
The Role of Imaging in Planning Therapy in HFrEF
Imaging techniques can be used to guide diuretic therapy, revascularisation decisions, intervention in valvular heart disease, device implantation and in the assessment of patients being considered for left ventricular assist device therapy.
Imaging in Guiding Diuretic Therapy
The goal of diuretic therapy in HF is to achieve euvolaemia, with the minimum possible dose. This is largely based on patients’ reporting of symptoms, basic clinical examination and a relatively crude estimation of the patients’ ‘dry weight’. Raised left ventricular filling pressures can be identified on TTE using a number of indices, such as the ratio of transmitral to annular early diastolic velocities (E/e’), tricuspid regurgitation velocity and left atrial volume index. These indices may provide a non-invasive tool to guide therapeutic decisions where there is clinical uncertainty. There is emerging evidence that adopting a TTE-guided approach to diuretic therapy is feasible and could lead to decreased HF mortality.20–22 The data are largely from small-scale pilot studies, although this is an interesting area for future research. However, given practical constraints, is likely to be reserved for the most challenging of clinical cases.
Imaging in Guiding Revascularisation Decision
Revascularisation in HF is indicated when angina persists despite optimal medical therapy. In patients with an ischaemic aetiology, imaging can be used to guide revascularisation decisions largely through the assessment of myocardial viability and the identification of hibernating myocardium.23 Dobutamine stress echocardiography is a widely validated method for the detection of hibernating myocardium, which relies on the ability to identify contractile reserve in response to low-dose inotropic agents. However, it is limited by being operator-dependent and not being available in some centres. SPECT with thallium-201 or technetium-99 labelled tracers offers valuable data regarding myocardial perfusion and viability. The uptake of perfusion tracers is dependent on myocardial perfusion and the integrity of the cell membrane. Hence, regions with preserved rest uptake are considered viable.
PET has been considered for many years as the gold standard for the assessment of viability using metabolic tracers. The spatial resolution is superior to that achieved with SPECT, and combination with attenuation correction allows quantitative analysis of regional myocardial blood flow and metabolism. Dysfunctional myocardial segments with higher FDG uptake compared with that of N-13 ammonia or rubidium-82 represent hibernating myocardium, while reduction on both perfusion and metabolism suggests scarring.24 Its main disadvantage is its limited availability and high associated costs. Finally, viability can be assessed on CMR by quantifying the transmural extent of scar, as detected on LGE sequences. If the transmural extent is <50%, then it is considered viable, and if it is >50%, then it is considered non-viable.23
Planning Intervention in Valvular Heart Disease
In patients with HFrEF and coexistent valvular heart disease, imaging plays a key role in determining the need and timing of valvular intervention. TTE is the key technique used to confirm the diagnosis, as well as to assess aetiology, mechanisms, severity and prognosis. TTE criteria for the definition of severe stenosis and regurgitation are addressed in specific guidelines, and are beyond the scope of this review.25 Transoesophageal echocardiography is used when TTE is of suboptimal quality, or when thrombosis, prosthetic valve dysfunction or endocarditis is suspected. Moreover, transoesophageal echocardiography has a role in assessing valve repairability. CMR can also be useful, especially in regurgitant lesions where the regurgitant fraction can be quantified on 2D phase contrast imaging.25 4D flow imaging is an emerging technology that allows time-resolved 3D velocity encoded phase-contrast imaging for the quantification of valvular flow.26 This may have a key role in quantifying regurgitant lesions, and assessing peak velocity through the aortic valve in aortic stenosis.27
Imaging in Device Therapy
HF guidelines recommend implantable cardioverter defibrillator implantation to reduce the risk of sudden cardiac death in patients with symptomatic heart failure (New York Heart Association class 2–3) and LVEF <35% after ≥3 months of optimal medical therapy who are expected to survive for at least 1 year with good functional status. Similarly, cardiac resynchronisation therapy (CRT) is indicated (class 1) in patients with symptomatic HFrEF in sinus rhythm with LVEF <35%, QRS duration >150 ms and left bundle branch block morphology despite optimal medical therapy, and is a class 2a recommendation in those with QRS duration of 130–149 ms or in non-left bundle branch block pattern with a QRS duration of >150 ms.28 Based on the current guidance, the role of imaging in patient selection largely focuses on accurate LVEF assessment. This is typically achieved with TTE, but CMR and nuclear studies can be used when poor acoustic windows are present.
CRT has been demonstrated to significantly reduce morbidity and mortality in HFrEF, but approximately 30–40% of patients are non-responders.29 As such, there may be an additional role for imaging in identifying potential non-responders to device therapy. Coexistent severe mitral regurgitation or RV dysfunction reduce the benefit achieved with CRT. Moreover, imaging can allow the assessment of dyssynchrony and the detection of myocardial scar, both of which have important implications for the response to CRT and defining the optimal LV lead positioning.
Several TTE and CMR techniques have been evaluated for the identification of mechanical dyssynchrony, including the presence of septal flash and apical rocking, tissue Doppler imaging and speckle tracking techniques to record myocardial velocities or strain, non-invasive and invasive ECG mapping, and vector cardiography. Radial dyssynchrony by speckle tracking imaging has been shown to be associated with EF and reverse remodelling response to CRT in patients with borderline QRS duration.30 Furthermore, LV myocardial work assessed with speckle tracking echocardiography has also been associated with survival in patients treated with CRT.31
Dyssynchrony can also be identified based on phase analysis of gated SPECT myocardial perfusion imaging.32 Phase analysis allows quantification of the temporal sequence of systolic ventricular wall motion, and is displayed as a colour coded histogram in which the y-axis represents the number of pixels, and the x-axis the phase angle. The latter corresponds to the relative sequence and pattern of ventricular contraction of each pixel within the LV blood pool. The mean phase angle is used to identify regional synchrony. This technique can be combined with scar burden assessment and lead concordance to predict the improvement in LV synchrony achieved with CRT. Machine learning-based algorithms show promise in phenotyping patients to identify those who will benefit from CRT by integrating clinical, electrical and imaging parameters, and are likely to be the focus of future studies.33
The location and extent of scarring identified on CMR or nuclear imaging adversely affect the chances of benefit from CRT, and assist in categorising ischaemic versus non-ischaemic aetiology of HF. Myocardial scar assessment can also help in risk prediction when determining the need for defibrillator functionality alongside CRT pacing.34 Finally, imaging techniques may also have a role in identifying optimal LV lead positioning. The LV pacing site has emerged as an important determinant of a favourable outcome following CRT implantation.35 Ideally, the LV lead should be placed at the latest activated segment (concordant lead positioning).28 Discordant lead positioning and placing at the site of scar are independent predictors of worse outcomes. Randomised controlled trials have demonstrated improved CRT response when using pre-implant imaging to guide the LV lead position towards the latest mechanically activated non-scarred myocardial segment.36 Studies applying TTE assessment of LV mechanical activation patterns and SPECT myocardial perfusion imaging have demonstrated an improved clinical outcome when the LV lead is located concordant to the latest mechanically activated region and separate from myocardial scar.
Furthermore, cardiac CT has the advantage of visualisation of the anatomical relationship between the coronary sinus tributaries and LV myocardium.37 An anatomical roadmap can be created by co-registering pre-implant 3D CT with fluoroscopic venography.38
Imaging in Patients Being Considered for Left Ventricular Assist Device Therapy
Patients with advanced HFrEF may ultimately receive a left ventricular assist device either as destination therapy or as a bridge to heart transplantation. Imaging plays an important role in their evaluation and guiding treatment both before and after device therapy. Prior to implantation, it is important to identify significant aortic valve disease or interatrial shunts.
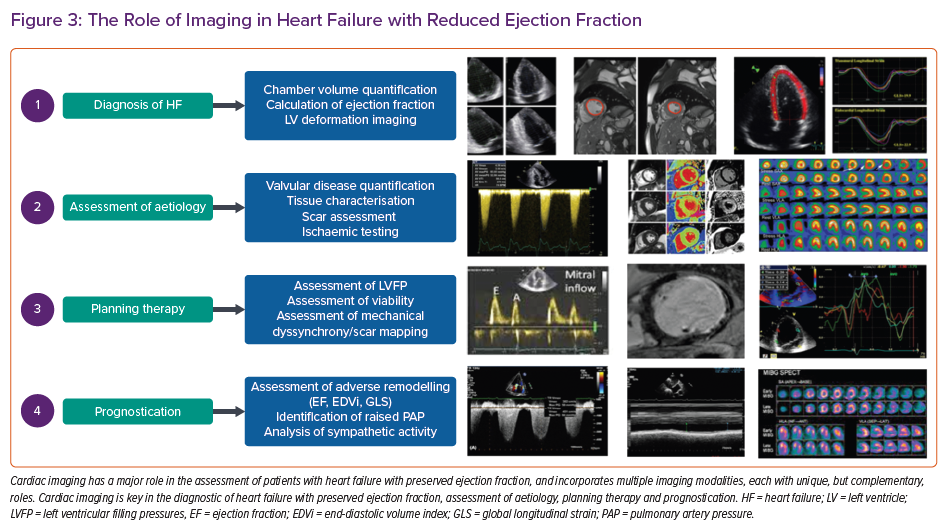
Additionally, the coexistence of RV dysfunction and TR need to be closely evaluated, as these patients may not respond as well to LV-only support.39 This can be achieved with TTE or CMR. After implantation, TTE remains the primary imaging technique, and is predominantly used to measure LV volumes, EF, LA and right atrial volumes. Furthermore, it can help to evaluate the effects of different pump speeds on LV filling, and LV and RV systolic dysfunction. Imaging is also used to identify complications associated with left ventricular assist device therapy, such as RV failure, thrombosis and driveline infection. Driveline infections are difficult to diagnose with conventional imaging. They affect approximately 18% of patients in the first year after implantation, and are associated with significant morbidity and mortality. TTE and CT angiography are commonly used, but the diagnostic accuracy is limited due to device-related scatter artefacts. More recently, FDG-PET/CT has been advocated for this indication and may have an important role (Figure 3).40,41
The Role of Imaging in Prognostication in HFrEF
Despite significant therapeutic advances in the treatment of HF, it remains a condition marked by progressive deterioration and premature mortality. Imaging can be used to identify patients with a worse prognosis. HF is characterised by ventricular remodelling, typically with increased ventricular volumes and perturbation in the normal elliptical LV chamber configuration, which is driven on a histological level by myocyte hypertrophy, apoptosis, myofibroblast proliferation and interstitial fibrosis. The degree and pattern of remodelling offers prognostic information and can predict clinical deterioration. LVEF assessment is influenced by the degree of remodelling, and is the most frequently measured and reported variable during follow-up.
The LV end-diastolic index at TTE has also been shown to be an independent predictor of survival.42 In the substudy of the VALIANT trial, LVEF, end-diastolic volume and end-systolic volume were each identified as independent predictors of the combined endpoint of death or HF hospitalisation.43 More recently, investigators have begun to explore the relevance of different patterns of LV remodelling on outcomes. Concentric remodelling, eccentric hypertrophy and concentric hypertrophy have been shown to exert progressively increased risk.44 GLS, as another marker of remodelling, also has a role in prognostication, and has been demonstrated to be superior to both LVEF and filling pressures in predicting outcome.45
The extent of LGE at CMR may also represent an important prognostic indicator. The number of segments with transmural infarct post-MI predicts the extent of subsequent LV remodelling, as evidenced by LV volumes and LVEF, and predicts the likelihood of functional recovery after revascularisation or medical therapy.46,47 CMR evidence of microvascular obstruction post-MI also predicts a greater likelihood of lack of functional recovery and future adverse events.48
Pulmonary hypertension in HF is common and is highly prognostic. A significant increase in mortality and hospitalisation has been reported in HF with TTE evidence of raised pulmonary artery pressures.49 Furthermore, pulmonary artery pressure is an independent predictor of the need for cardiac transplantation.50 In addition, the pulmonary artery pulsatility index, defined as the ratio of pulmonary artery pulse pressure to right atrial pressure, serves as a marker of RV dysfunction, and has been demonstrated to be an independent predictor of hospitalisation and death.51 Detection of secondary pulmonary hypertension is typically achieved based on 2D TTE. Pulmonary artery pressure is assumed to be equal to RV systolic pressure, which can be estimated from the maximum velocity (using the Bernoulli’s equation) of the TR jet, as assessed by Doppler imaging.
Advanced HF is characterised by a multitude of molecular events, as such radionuclide imaging is well suited. Autonomic dysfunction has been shown to increase the risk of death in patients with HF. Cardiac MIBG imaging enables non-invasive and quantitative assessment of cardiac sympathetic innervation.52 Increased sympathetic activity reflects worse HF, and a survival link has been identified.53 Assessment of myocyte apoptosis using 99 mTc annexin 5 is another technique that may emerge in the future. This can identify early rejection in heart transplantation recipients, as well as patients with progressive worsening of symptoms in dilated cardiomyopathy.54 Imaging of matrix metalloproteinase activity may also play an important role in defining the process of LV remodelling after MI, and warrants further investigation.
Conclusion
HFrEF represents a major health burden and, despite advances in treatment, is associated with significant morbidity and mortality. Cardiac imaging has a key role in the diagnosis, assessment of aetiology, treatment planning and prognostication of patients with HFrEF. TTE and CMR remain the most commonly used modalities for accurate assessment of LV volumes and function, quantification of valvular disease, identification of scar, and tissue characterisation. Cardiac imaging is a rapidly evolving area, and with the emergence of advanced technologies, has an increasingly important role in treatment planning and prognostication that will continue to grow as technologies develop further.