The increasing prevalence of heart failure requires the establishment of new imaging techniques that are able to diagnose early and guide treatment. With an estimated 825,000 new cases annually in the US, the heart failure burden will continue to rise and is expected to exceed 8 million by 2030.1
Echocardiography
Left Ventricular Function: The New Definition of Mid-Range Ejection Fraction Heart Failure
Echocardiography is a first-line bedside tool for the diagnosis of heart failure patients and the assessment of prognosis,2,3 with ejection fraction (EF) having been the cornerstone of heart failure definition.2 During the last decade, left ventricular (LV) EF has been employed to describe the new entity of mid-range EF heart failure (HFmrEF) as being an LVEF of 41–49 %.3 It is now clear that patients with HFmrEF represent a grey area,4,5 with recent data from major trials6,7 demonstrating that patients with HFmrEF behave similarly to those with heart failure with reduced EF (HFrEF), in terms of both prognosis and response to therapy. As a result of the new definition, more light has been shed on diastolic dysfunction and its role in the development of heart failure.
Diastolic Assessment and the Value of Exercise Testing
By returning to the basics, two important echocardiographic features are important in heart failure patients: an accurate evaluation of diastolic function8 and the value of exercise testing.9,10
Evaluation of diastolic dysfunction at rest
Accurate assessment of the degree of diastolic dysfunction is a prerequisite for a heart failure assessment. During diastology assessment, mitral E and annular E’ velocities should be recorded as well as peak velocity of tricuspid regurgitant jet from multiple windows.8 The key structural alterations during diastolic dysfunction in patients with normal EF are:8
- left atrial volume index >34 ml/m2, or left ventricular mass index ≥115 g/m2 for males and ≥95 g/m2 for females;
- average E/e’ >14;
- annular e’ velocity : septal e’ <7 cm/s and lateral e’ <10 cm/s; and
- peak tricuspid regurgitant velocity >2.8 m/s.
Pulmonary pressures are of crucial importance as they are an indirect marker of significant diastolic dysfunction in patients with even normal LVEF.11 When the ejection fraction is reduced or where there is a confounding factor such as volume overload, other parameters are useful for the assessment of LV filling pressures.
Exercise stress test and diastolic dysfunction
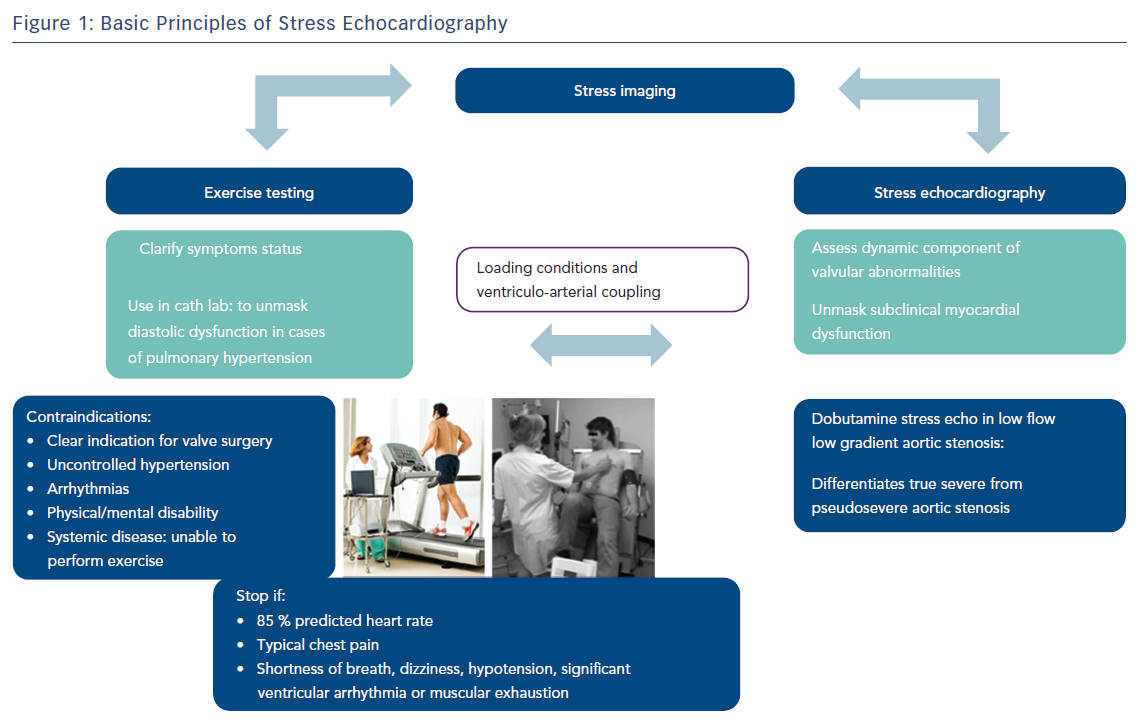
In patients with shortness of breath and grade 1 diastolic dysfunction at rest, diastolic stress testing is crucial in diagnosis and prognosis (Figure 1). It is usually performed with a treadmill or supine bicycle. The test is considered positive if all the following three criteria are met during exercise:9,10
- average E/e’ >14 or septal E/e’ ratio >15;
- peak tricuspid regurgitant velocity >2.8 m/s; and
- septal e’ velocity <7 cm/s.
Strain Echocardiography
Impaired global longitudinal strain (GLS) is common among HFpEF patients, indicating the presence of covert systolic dysfunction despite normal LVEF.13 Furthermore, impaired strain has been associated with biomarkers of wall stress and collagen synthesis and diastolic dysfunction.13 Longitudinal ventricular strain has been correlated independently to peak VO2 in patients with reduced and preserved EF and has been superior in identifying patients with reduced exercise capacity. There is a significant relationship between diastolic function and GLS, confirming a coupling between diastolic and longitudinal systolic function in HFpEF.14
Unique strain patterns may guide the diagnosis in certain cardiomyopathies such as the apical sparing of longitudinal strain in amyloidosis (Figure 2).15 Furthermore, strain has emerging and valuable input in the early detection of chemotherapy-related cardiotoxicity.16 Peak systolic GLS appears to be the best measure. A 10–15 % early reduction in GLS by speckle-tracking echocardiography during therapy appears to be the most useful parameter for the prediction of cardiotoxicity, defined as a drop in LVEF or heart failure. Latest findings raise awareness among physicians to perform early strain echocardiography in patients on chemotherapy such as anthracyclines. As demonstrated in the literature,17 GLS greater than −17.45 % obtained after 150 mg/m2 of anthracycline therapy is an independent predictor of future anthracycline-induced cardiotoxicity.
Left atrial strain is also an emerging imaging index that highlights stiffness, and the latter has been associated with left atrial dysfunction and its associated prognosis in patients with heart failure.18 It also helps indirectly to raise a suspicion of fibrosis, especially in patients with a predisposition to develop AF.19
Right Heart
Imaging modalities have demonstrated significant progress in terms of imaging of the right heart. We employ new software for right ventricular reconstruction, while right atrial dimensions either by measuring the volume or by measuring more complex indices such as the sphericity index, have been associated with functional outcomes and a particular prognosis in patients with pulmonary hypertension (Figure 3).20
In patients with heart failure, as mentioned above, the assessment of pulmonary pressures either at rest or during exercise are of prognostic value. Furthermore, right ventricular area strain is now associated with functional outcomes and with prognostic markers in patients with pulmonary hypertension.21
Multidetector CT
Cardiac CT imaging aims to employ the latest technology22,23 together with ECG gating in order to reduce scan time and radiation exposure. Multidetector CT (MDCT) can measure infarct size with accurate correlation to measurements obtained through cardiac MRI (CMR) and can be a valuable alternative to CMR in patients with non-MRI conditional pacemakers or defibrillators.22,24
Coronary calcium scan is a reliable test to screen for coronary artery disease and has been integrated in clinical practice as a risk stratification imaging marker, especially in subgroups such as renal patients.23 Furthermore, dual-source CT can evaluate LV dyssynchrony, myocardial scar and coronary venous anatomy in patients undergoing cardiac resynchronisation therapy.24 Time to maximal wall thickness and inward wall motion measured with MDCT has been associated with the prediction of major adverse cardiac events.25
One step beyond the morphological assessment of coronary arteries, dual-source CT combines coronary artery anatomy and myocardial perfusion imaging (MPI). An emerging modality, stress myocardial CT perfusion imaging with adenosine may detect haemodynamically significant coronary lesions,26,27 with data comparable to that from CMR and single-photon emission CT (SPECT). Wang et al. performed a 30-patient feasibility study of adenosine-stress dynamic MPI with 128-MDCT dual-source CT for detecting flow-obstructing stenosis.26 A larger and multi-centre study by Meinel et al. proved that myocardial perfusion CT has incremental value in predicting clinical risk factors and detecting coronary artery stenosis compared with coronary CT angiography CCTA.28
Computational fluid dynamics measures fractional flow reserve (FFR), and this method (CT-FFR) has also been validated against invasive FFR in patients with stable coronary artery disease.29,30 Adding FFR measurement may add in favour of invasive angiography, as an CT-FFR of ≤0.80 has been a better predictor of revascularisation or major adverse cardiac events than severe stenosis on a coronary CT angiogram.30
In electrophysiology, 3D reconstruction of cardiac structures from CT data sets has revolutionised the understanding of left atrial anatomy and greatly facilitated the development of novel atrial fibrillation ablation approaches.19 CT also tends to guide pulmonary vein isolation during ablation of atrial fibrillation procedures.31
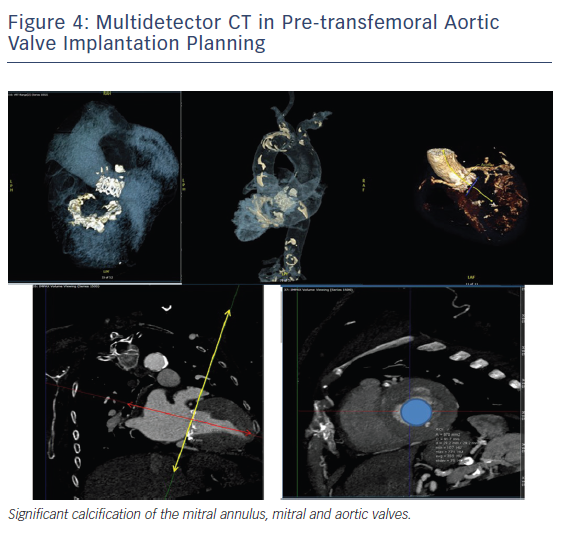
Finally, CT has a key role in evolving interventional procedures such as trans-catheter aortic valve implantation, where annulus can be accurately measured to size the valve as well as the distance of coronary ostia.32 Accurate aortic annulus measurement is critical to identify the appropriate valve prosthesis size (Figure 4). Aortic root MDCT also allows prediction of the angulation of the root angiogram, which facilitates the angiographic procedure by reducing the number of root injections.33 MDCT is also fundamental in the evaluation of size, tortuosity and calcification of peripheral arteries, from the aortic valve down to the femoral arteries, and guides the selection of appropriate-size catheters during the procedure.34
Cardiovascular Magnetic Resonance
CMR has emerged as a superior imaging modality in the non-invasive assessment of heart failure, adding to the diagnosis, when conventional imaging modalities such as echocardiography fail to specify the aetiology, but also CMR has a vital role in prognosis and in guiding treatment decisions.35,36
CMR has several advantages when compared with other imaging modalities, due to accuracy, reproducibility, larger field of view, no radiation, and the ability to characterise myocardial tissue. It allows comprehensive evaluation of ventricular size and function to establish the cause of heart failure, determine myocardial perfusion and viability and mechanical dyssynchrony, and evaluate pericardial disease.35 CMR is recognised as the gold standard method for the quantification of ventricular volumes.
Velocity-encoded CMR37 may quantify mitral diastolic flow and diastolic longitudinal myocardial lengthening velocity of the LV base. CMR precontrast T1 of infarcted tissue is significantly correlated with regional diastolic circumferential strain rate.38 For patients with intermediate pretest likelihood of CAD, vasodilator stress CMR is used to detect perfusion defects, and dobutamine stress CMR is used to assess for wall motion abnormalities, combined with quantitating deformation with strain-encoded CMR to improve accuracy over visual assessment on cine imaging.39,40
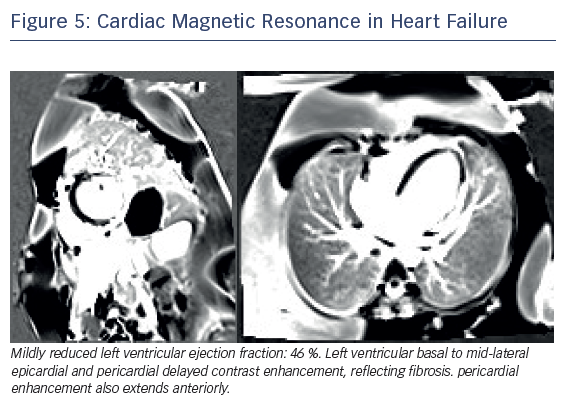
Late gadolinium enhancement (LGE) imaging is fundamental to differentiate between ischaemic and non-ischaemic cardiomyopathy. Full-thickness infarcts as assessed by LGE-CMR had been shown to correlate inversely with the likelihood of segmental and global functional recovery post revascularisation.40,41 Dilated non-ischaemic cardiomyopathy is characterised by mid-wall distribution of LGE, and the presence independently predicts major adverse cardiovascular events, including hospitalisation for decompensated heart failure, sudden and non-cardiac sudden death, malignant ventricular arrhythmias and all-cause mortality (Figure 5).42
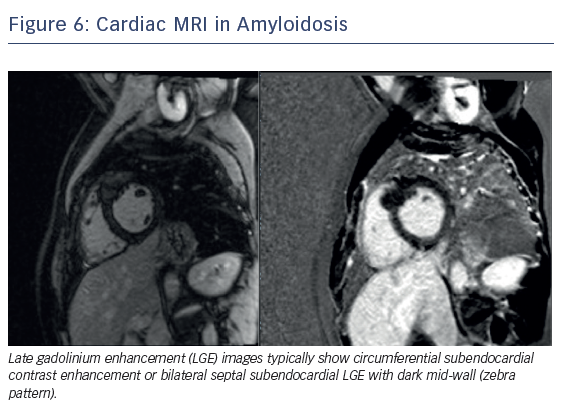
CMR is the primary non-invasive imaging modality for the assessment of suspected myocarditis in clinically stable patients, by evaluating LV function.43–45 In patients with heart failure and possible iron overload, CMR with T2* provides definitive diagnosis. CMR in cardiac amyloidosis typically reveals increased myocardial gadolinium enhancement (global, sub-endocardial or transmural), short T1, rapid blood pool wash-out and suboptimal myocardial nulling.15 As a summary, CMR aids in diagnosis, risk stratification, treatment guidance and prognosis from the wealth of information obtained through myocardial tissue characterisation using T1, T2 and T2* mapping sequences and quantitative volumetric analysis (Figure 6). Future research in 3D LGE, strain imaging and diffusion tensor CMR is ongoing and offers promise to improve diagnostic accuracy and reproducibility.
Molecular Imaging
In heart failure, the autonomic nervous system is disrupted, initially with upregulation of the sympathetic nervous system to compensate for a failing heart and, over time, downregulation of cardiac receptors. Radionuclide imaging, using either PET or single-photon emission CT (SPECT), has been the modality most commonly used for human molecular imaging, Molecular imaging allows tissue characterisation in myocardial disorders at molecular, subcellular and cellular levels. It has the potential for assessing the severity of heart failure and for monitoring treatment effects. 123I-labelled MIBG, as a marker of adrenergic neuron function, could play an important role in the risk stratification of heart failure patients.46 A major focus has been on the ability of 123I-MIBG imaging to risk-stratify patients for risk of ventricular arrhythmic sudden cardiac death, and thereby guide the use of an ICD.46 As cardiac autonomic innervation is more closely related to underlying mechanisms of arrhythmias than LVEF, MIBG imaging should better determine the need for an ICD.46,47
Furthermore, emerging studies highlight the role of coronary flow reserve with stress myocardial perfusion PET as an independent predictor of diastolic dysfunction and adverse effects, especially hospitalisation of heart failure patients with preserved EF.48 The presence of both coronary microvascular and diastolic dysfunction was associated with a markedly increased risk of heart failure events.
Hybrid Imaging and 3D Printing
Cardiac hybrid or fusion imaging combines imaging modalities, and this advanced imaging technique sheds light on anatomical and functional information. It provides additional information in heart failure patients who are complex patients requiring imaging of coronary arteries, assessment of myocardial viability, and evaluation of valvular abnormalities in combination with haemodynamic alterations. Hybrid imaging with CT can guide interventional procedures like revascularisation, cardiac resynchronisation therapy and structural heart valve disease interventions (transcatheter aortic valve implantation, MitraClip, left atrial appendage occlusion in heart failure patients with AF).49,50 SPECT/CT and PET/CT may improve the diagnostic accuracy for ischaemic heart disease in patients with heart failure, allow the determination of functionally relevant coronary stenosis and guide targeted revascularisation in the elevated-risk population. Based on hybrid imaging, 3D printing allows careful planning of complex procedures and aims to minimise complications and improve outcomes.51
Clinical Perspective
- This review highlighted the latest advances in cardiac imaging and, specifically, their implementation in the diagnosis and management of heart failure.
- The clinical decision pathway is very well described in the latest guidelines.3
- Echocardiography is the first-line imaging modality, especially because it can be portable even in the intensive care environment. It may be complemented by other modalities, chosen according to their ability to answer specific clinical questions and taking account of contraindications to and risks of specific tests.
- The reliability of clinical outcomes and the management strategy are highly dependent on the imaging modality, the operator and centre experience. Guidelines3 are bringing together the latest studies and indicate clinical algorithms on when and how each imaging modality may be used. Despite this, there is an ongoing need for randomised clinical trials to specify indications and limitations of each modality.