Heart failure (HF) is a major national and international pandemic with a prevalence of more than 5.8 million in the US and over 26 million worldwide. It is the leading cause of hospitalisation and healthcare costs in the US and it accounts for more than 1 million hospitalisations annually. In the US, up to 25% of patients hospitalised with HF are readmitted within 30 days.1 The financial burden of acute decompensated heart failure (ADHF) on the healthcare system was estimated at $30.7 billion in 2012 with a projected threefold increase by 2030 leading to a total of $160 billion total annual cost.2
HF has multiple aetiologies and the goal of therapy is aimed not only at resolving the root cause but providing symptomatic relief in light of medication failure. The major causes of HF are distinguished between those involving heart muscle disease including those with ischaemic and non-ischaemic cardiomyopathies and those with structural abnormalities.
The adult with CHD (ACHD) or structural heart disease (SHD) represents a large proportion of the HF epidemic. With congenital heart disease (CHD) present in 0.8% to 1.0% of live births, and the advances in medical and surgical therapies for children with CHD, the growing proportion of these patients reaching adulthood now exceeds 85% with an estimated 1.2 million patients in this group in the US.3 SHD essentially encompasses a wide range of non-coronary cardiac disease processes, some of which include valvular disease, paravalvular leak and septal defects. Historically, the mainstay of therapy has been cardiac surgery for these types of disease, but with the advent of new minimally invasive technologies and techniques, structural intervention has changed the way this subset of patients receives treatment and has resulted in newer therapies that can be used in patients with HF. Identification of the mechanisms responsible for HF is essential in the management of patients with SHD because these can become targets for therapies.4,5
Over the past decades, the rapid development of successful transcatheter procedures has led to interventional procedures becoming the primary treatment for many forms of SHD. In this review, we focus on structural interventions for the treatment of patients with HF due to both acquired and congenital defects.
Acquired Defects
Aortic Stenosis
Aortic stenosis (AS) is one of the most common valvular pathologies with sclerosis being present in 21–26% of people aged 65 years or older. The most common aetiology is senile or calcific aortic stenosis with younger people either being diagnosed at birth or with early degenerative valves (i.e. bicuspid valves).6,7
Patients are usually asymptomatic until late in the course of the disease when they have a high risk of acute decompensation especially when associated with congestive HF.8 Survival analyses have demonstrated that the interval from the onset of symptoms to the time of death is about 2 years in patients with HF.9,10 Stewart et al. reported that among symptomatic patients with moderate-to-severe AS who had been treated medically, mortality rates after the onset of symptoms were about 25% at 1 year and 50% at 2 years.11 More than 50% of deaths were sudden.
Transcatheter interventions are at the forefront of treatment options for severe AS. Balloon aortic valvuloplasty (BAV) has been a long-standing option for younger patients with bicuspid valves with current practice guidelines recommending it for symptomatic young adults without severe calcification and less than moderate regurgitation with a peak-to-peak gradient of 50 mmHg, or asymptomatic young adults with ST-T changes and a peak-to-peak gradient of 60 mmHg.11,12 BAV is also a viable option for patients who have advanced HF who are haemodynamically unstable or as a palliative approach when the effect is time limited.
Since the onset of transcatheter aortic valve replacement (TAVR), multiple trials have supported its use in patients with severe AS in various stages of their disease process. The Placement of AoRTic TraNscathetER Valve Trial (PARTNER) trials have studied TAVR in patients with severe AS and high-, moderate- and low-risk patients, respectively. The results of the trials have all favored TAVR as an option over surgical AVR and has now become one of the most promising advances in structural heart disease. Recent data have shown a decline in hospitalisation from HF from 15.9% pre-TAVR to 14.2% in the year after TAVR with the majority of hospitalisations being due to non-cardiac causes.13 In a retrospective study performed by Muratori et al., TAVR patients showed an average improvement in New York Heart Association (NYHA) class of ≥1 at 6 and 12 months post-implantation with marked reduction in the degree of LV diastolic dysfunction regardless of baseline LV ejection fraction. There was improvement in LV end-systolic volume and LV mass index at 6 and 12 months.14
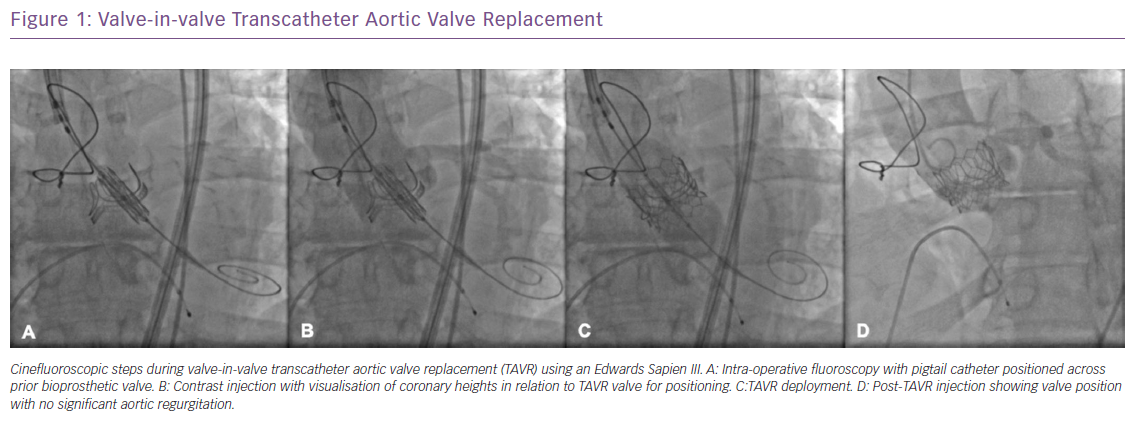
With newer technologies, such as the CoreValve Evolut Pro (Medtronic) self-expanding valve and the enhanced paravalvular sealing effect of the third-generation SAPIEN 3 valve, TAVR has become a viable option for bicuspid aortic valve stenosis and can effectively treat AS before the onset of worsening clinical symptoms and deterioration. The benefits of TAVR extend further to a subset of patients who have had prior valve replacement. As bioprosthetic valves degenerate, recurrent severe AS or regurgitation can progress with limited means of intervention. Transcatheter valve in valve (ViV) TAVR procedures have been performed in patients who are considered high surgical risk with excellent results and this technology is being further studied in the low surgical risk population (Figure 1).
Mitral Stenosis
Mitral stenosis (MS) is characterised by a reduction in the mitral valve (MV) orifice area causing obstruction of left ventricular (LV) inflow and decreased LV filling. Although its prevalence has declined, especially in developed countries, it is still an important cause of morbidity and mortality. Rheumatic fever still remains the predominant cause of MS worldwide accounting for about 10% of native valve disease, but the incidence of degenerative MS has been more pronounced in developed countries.
MS caused by rheumatic fever occurs due to post-rheumatic commissural fusion usually in adolescents. The calcified changes of the mitral annulus cause degenerative MS most commonly seen in the elderly. The decrease in the mitral valve (MV) orifice area causes stagnation of blood proximal to the MV that results in elevated left atrial, pulmonary venous and pulmonary artery pressures ultimately leading to HF.
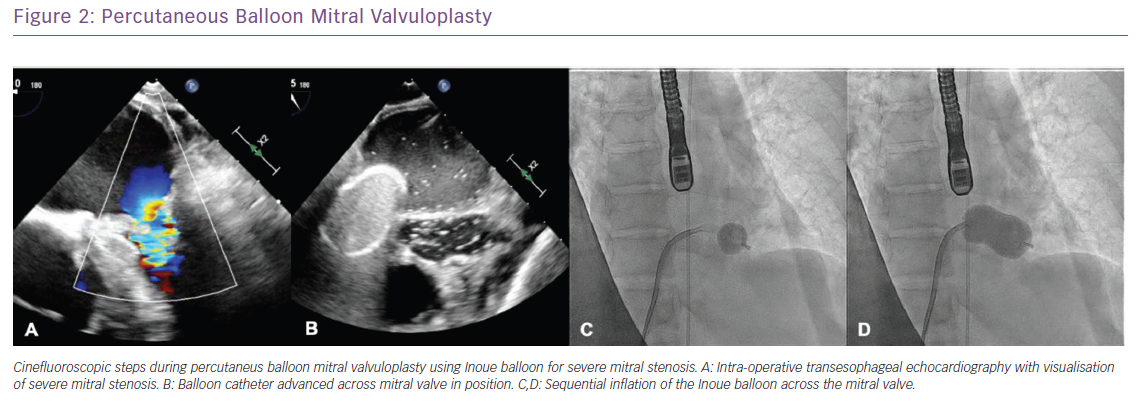
Mitral valve intervention has been the mainstay of therapy for symptomatic patients with the option of balloon valvuloplasty or surgical valve replacement.15 Percutaneous mitral balloon valvuloplasty (PMBV) is the preferred treatment for patients with favourable anatomy based on their Wilkins score which takes into account MV leaflet mobility, thickening, calcification and subvalvular thickening with a score £8 favouring PMBV.16 Recent data have shown that patients with suitable anatomy for PMVB exhibited a 75% sustained durability over a 23-year span with median 8.3-year follow-up. Meneguz-Moreno reported that during follow-up, the majority of patients (93.1%) had improvement in NYHA classification in the first post-procedural year with 13% developing NYHA class III/IV symptoms associated with poor outcomes; 19.1% of patients had a primary endpoint, including 0.6% who died, 8.8% who underwent mitral valve surgery and 10% who required another PMBV (Figure 2).16
In the case of degenerative MS, patients tend to be older with higher surgical risks and significant calcification making PMBV suboptimal leaving them with few treatment options.17 Transcatheter mitral valve replacement (TMVR) is an evolving therapy where the mitral valve is accessed via a transseptal or transapical approach and a new valve is deployed reducing the risks of a more aggressive surgical approach. It has allowed for an expanded treatment option for patients with degenerative MS with the ability of valve replacement in patients with significant mitral annular calcification (MAC). Complications vary and include compromised left circumflex artery, severe bleeding and atrioventricular rupture but TMVR has been a promising field for advancement especially for the symptomatic patients with recurrent HF admissions deemed non-surgical candidates.18
Mitral and Tricuspid Valve Regurgitation
Long-standing HF leads to distortion of the structural integrity of the heart causing LV dilatation and dislocation of the papillary muscles and chordae tendinae cause worsening mitral regurgitation (MR) from impaired coaptation of the mitral valve leaflets. Such secondary (functional) mitral regurgitation increases the severity of volume overload and has been strongly associated with a reduced quality of life, an increased rate of hospitalisation for HF and shortened survival.19
Guideline-directed medical therapy and cardiac resynchronisation therapy may provide symptomatic relief, improve LV function and, in some patients, lessen the severity of MR but whether the correction of secondary MR improves the prognosis among patients with HF is unknown.20 Although mitral valve surgery is curative for primary (degenerative) MR, neither surgical repair nor surgical replacement of the mitral valve has been shown to lower the rate of hospitalisation or death associated with secondary MR and both procedures confer a substantial risk of complications.21
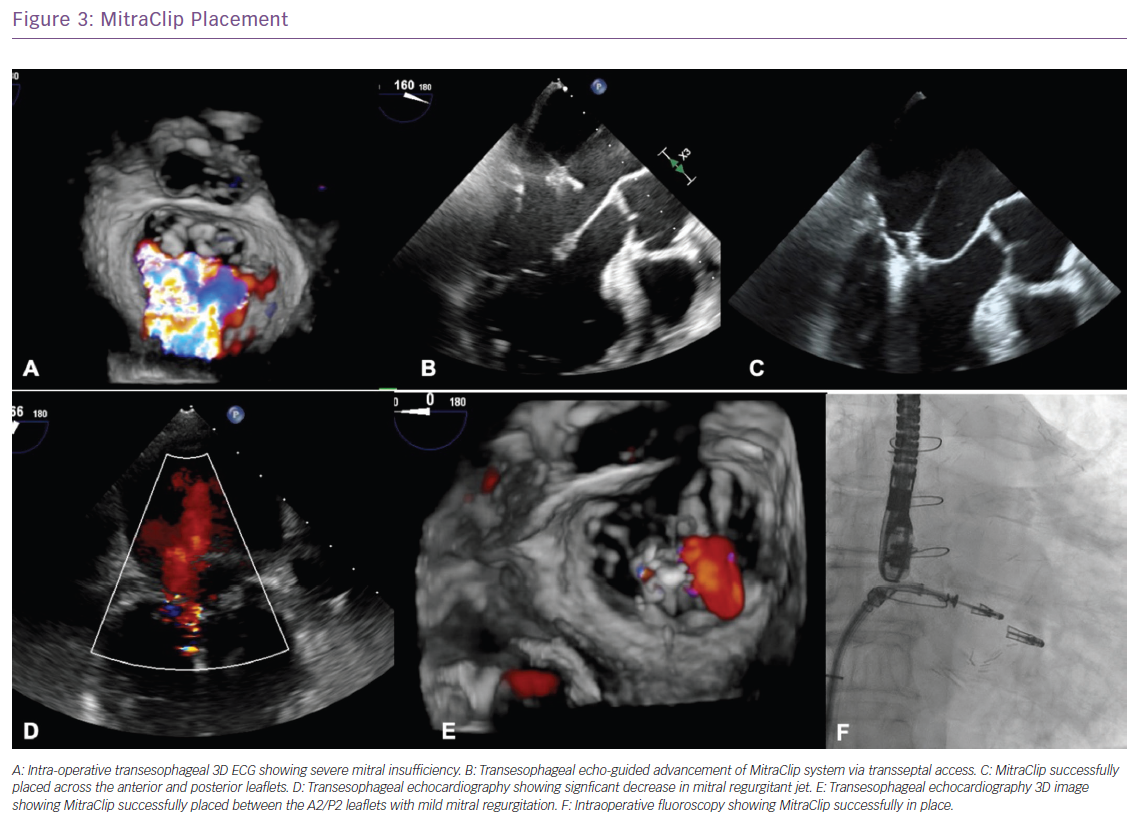
One of the most innovative advances in SHD stems from the recently published Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation (COAPT) trial which involved patients with HF and moderate-to-severe or severe secondary MR who remained symptomatic despite the use of maximal doses of guideline-directed medical therapy; transcatheter mitral-valve repair with MitraClip (Abbott) resulted in a lower rate of hospitalisation for HF and lower all-cause mortality within 24 months of follow-up compared with medical therapy alone.22 The study found that the annual rate of all-hospitalisation for HF was significantly lower in the device group at 35.8% compared with 67.9% in the control group with a number needed to treat of 3.1. All-cause mortality benefit was also significantly lower in the device group at 29.1% versus 46.1% (HR: 0.62, p<0.001) with the number needed to treat to save one life within 24 months being five.22 The MitraClip has also been approved for degenerative (primary) MR in patients who are considered high risk for surgery (Figure 3).
With the advances in transcatheter therapies, new innovations for the treatment of tricuspid regurgitation has emerged MitraClip device has been used as an off-label treatment option as reported by Nickenig et al.23 In their report, patients with HF symptoms and severe TR were randomised to medical therapy versus MitraClip for chronic, severe TR and with a successful implantation rate of 97%, they found the grade of TR to be reduced by at least one grade in 91% with significant reduction in echo parameters associated with severe TR. Newer technologies and studies are ongoing for TTVR repair including a large Triclip study creating a ‘triple-orifice technique’ of clipping between the septal and anterior, as well as the septal and posterior leaflets, while avoiding grasping between the posterior and anterior leaflet.24 The Trialign and TriClinch annuloplasty systems are also showing promises to alternative valvular interventions.24
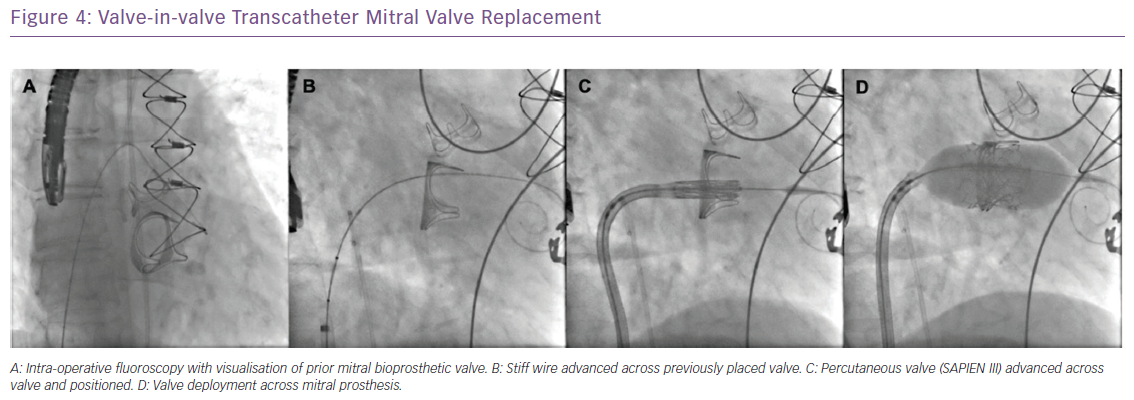
Similar to patients with failed bioprosthetic aortic valves, ViV transcatheter approaches have also been used with great success in patients with dysfunctional mitral and tricuspid bioprosthetic valves using the Edwards Sapien 3 valve (Figure 4).
Percutaneous Pulmonary Valve Implantation
Transcatheter pulmonary valve replacement (tPVR) is one of the more recent developments in the treatment of SHD and HF and has evolved as an attractive alternative to surgery in patients with dysfunctional right ventricle-pulmonary artery conduits or bioprosthetic valves. The most common indication of pulmonary valve implantation is residual right ventricular outflow tract (RVOT) lesion after repair of CHD that can be stenotic, regurgitant or mixed.
The advent of tPVR with Melody and Sapien valves has dramatically altered the management of these patients. Patients with tetralogy of Fallot who have had valved conduits or bioprosthetic valves placed and patients who have had the Ross procedure, where the pulmonary valve is autotransplanted to replace the diseased aortic valve and a valved conduit is used between the right ventricle and pulmonary artery, are the two largest adult patient groups who are receiving these valves. Even though tPVR is not currently a standard indication in adults with native RVOT dysfunction, many centres have performed tPVR in a select group of adults who have a native outflow tract (post transannular patch repair of tetralogy of Fallot) that is an appropriate size or one that can be altered to a suitable size by insertion of multiple stents.25
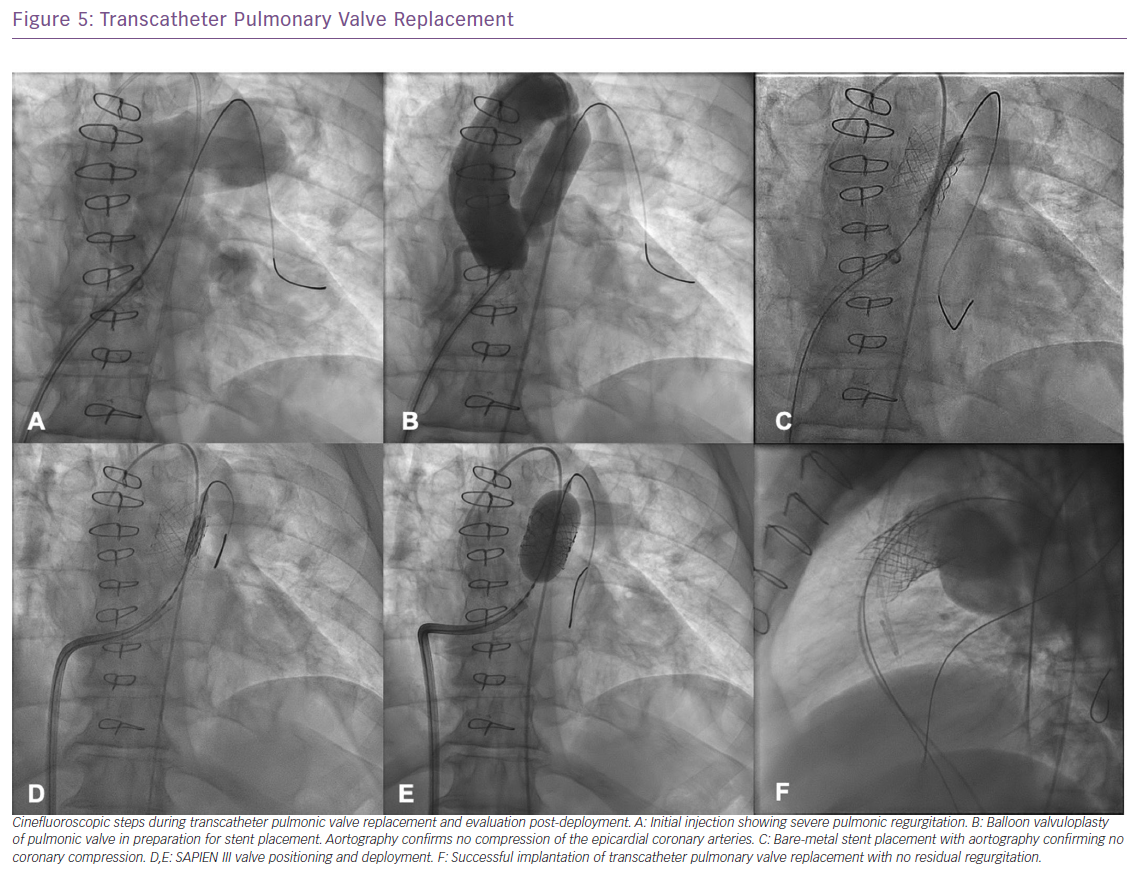
It is crucial that only appropriate patients are selected for pulmonary valve replacement and the guidelines for surgical and transcatheter pulmonary valve replacement have continued to evolve over the past decade.26–30 Pulmonary valve replacement should ideally be performed before RV function declines. In addition to clinical indications, several anatomical criteria need to be fulfilled to qualify for tPVR with the ideal anatomy being a uniform diameter from RVOT to pulmonary artery with adequate main pulmonary artery length to avoid stenting into the pulmonary artery bifurcation (Figure 5). When the conduit is placed on the anterior surface of the heart, coronary branches may pass directly beneath it, and they may be compressed by the placement of the stented valve and distension of the conduit.7 If no evidence of coronary compression is noted, pre-stenting of the RVOT is performed to create an appropriate landing zone for the transcatheter valve. Pre-stenting the landing site has significantly improved the survival of the implant, minimising stent fracture which affected 23% of the initially reported cases of the Melody valve (Medtronic).25
Outcomes were recently reported from the COngenital Multicenter Trial of Pulmonic VAlve Regurgitation Studying the SAPIEN InterventIONal THV (COMPASSION) trial showing a significant improvement in NYHA functional class in 93.5% of patients with freedom from all-cause mortality at 3 years being 98.4%.31 Similar findings were reported from the Melody Trial with functional outcomes improving post tPVR.31 The patients enrolled were NYHA class I (17%), class II (67%) or class III (16%) with median RV systolic pressure of 74 mmHg. At 12 months, the median RV pressure decreased to 51 mmHg and there was a significant improvement in NYHA functional class with patients. At 6-month, 1-year and 2-year follow-up, patients continued to see improvement in their functional status with only 1–2% being class III or IV at the median follow-up of 4.5 years.32
Paravalvular Leak
Prosthetic paravalvular leaks (PVLs) are a well-recognised complication of both surgical and transcatheter valve replacement. Various series have demonstrated that 5–15% of all surgical valve replacements are complicated by some form of PVL and 40–70% of patients who undergo transcatheter aortic valve replacement.33–35
Significant PVL can lead to major clinical and haemodynamic consequences and it can affect long-term survival. Symptoms may range from a decrease in functional class to severely decompensated CHF and/or haemolysis. Furthermore, persistent PVL has been shown to increase mortality.35 Reoperation for PVL is associated with a high recurrence rate and carries significant morbidity and mortality risks.
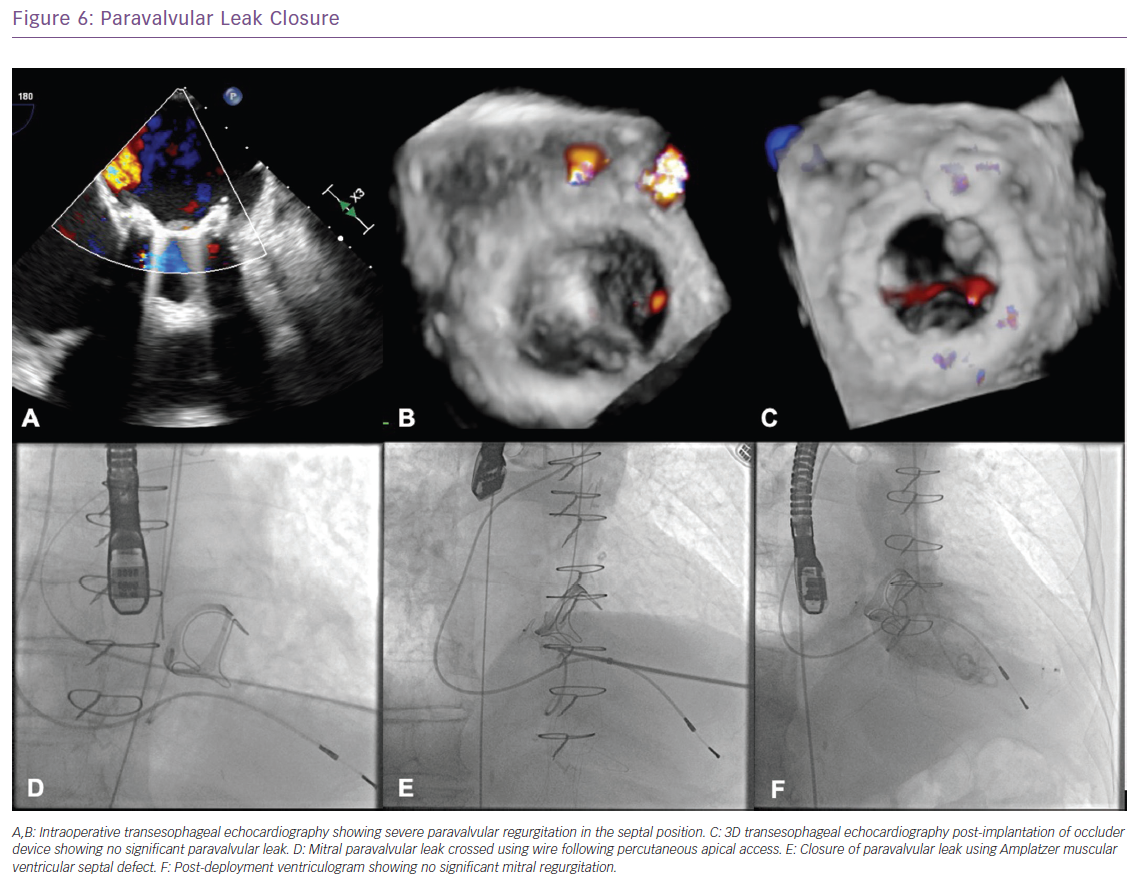
With the advent of recent developments in percutaneous interventions for the treatment of SHD, efforts have been made to seal PVL percutaneously by delivering occluders to the site of leak, preventing or reducing the amount of regurgitation. The percutaneous approach is now an established therapy for symptomatic patients with PVL and is frequently considered as a primary therapy for eligible patients and can be performed via a retrograde transaortic, anterograde transseptal or transapical (TA) approach (Figure 6).36
In the US, there are no approved devices that are designed specifically for PVL closure and Amplatzer occluders are the most commonly used off-label devices (Figure 1). For large PVLs, multiple devices might need to be used either sequentially or simultaneously. Outside the US, there are devices designed specifically for closure of paravalvular mitral leaks, such as Occlutech PLD devices and Amplatzer Vascular Plug-III Figure 1).37,38
Congenital Defects
Pulmonary valve stenosis
Pulmonary valve stenosis (PVS) is a common congenital lesion found in children that may escape detection in childhood and present in adulthood with significant stenosis. It is typically caused by commissural fusion resulting in diminished valve orifice and increased RV afterload. Symptoms may vary from mild exertional dyspnoea to signs and symptoms of right HF, depending upon the severity of obstruction and the degree of myocardial compensation.
Balloon pulmonary valvuloplasty has evolved to be the procedure of choice for the treatment of pulmonary valve stenosis. Indications for intervention on isolated pulmonic stenosis include peak gradient >50 mmHg or mean gradient >30 mmHg in symptomatic patients. In asymptomatic patients, intervention may be considered with peak gradient greater than 60 mmHg or mean gradient greater than 40 mmHg.37 Balloon pulmonary valvuloplasty has uniformly excellent results in all age groups, with results comparable to surgical valvotomy, it has low recurrence risk and can be easily repeated if necessary. The double-balloon technique, which uses two smaller balloons from each femoral vein, has also been applied to pulmonary valve stenosis with excellent results.39
Atrial Septal Defects
Atrial septal defect (ASD) is the most common form of CHD to escape detection in childhood comprising between 20 and 40% of all newly diagnosed CHD in adults. Under normal physiological conditions, flow through an ASD occurs from left to right at a variable degree depending on the defect size, ventricular compliance and the pulmonary vascular resistance. Excessive flow through the defect can eventually result in chronic right heart volume loading state, eventually leading to long-term complications in the second or third decade of life including premature death, atrial arrhythmias, reduced exercise tolerance, RV diastolic and systolic failure, LV diastolic failure and pulmonary arterial hypertension.40,41 Therefore, early closure of haemodynamically significant defects is recommended.
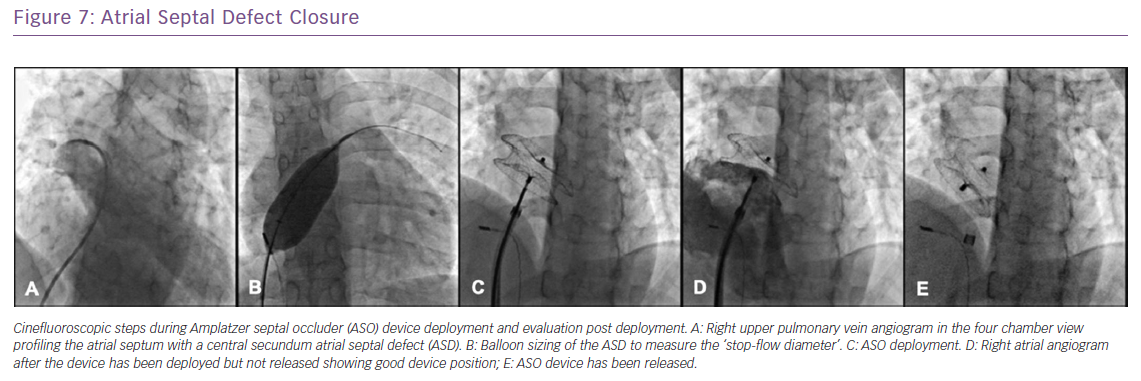
Percutaneous closure of secundum ASD is currently the standard of care with a success rate exceeding 98% among patients who have suitable margins and defect size. Depending on the experience of the operator, the procedure may be done under conscious sedation using intracardiac echocardiography (ICE) or under general anaesthesia with the use of transesophageal echocardiography (TEE) to define the anatomy of the defect and to guide device deployment. The choice of device depends on its availability, the exact anatomy of the defect as well as operator preference. In the US, two devices are approved for this indication: the Amplatzer Septal Occluder device (St Jude Medical) and the Gore Helex Septal Occluder (Gore Medical). Multiple other devices are available internationally, the most commonly used is the Occlutech Figulla Flex II device (Occlutech), a double-desk device similar in design to the Amplatzer septal occluder (Figure 7).
Ventricular Septal Defect
Ventricular septal defects (VSD) comprise as many as 10% of SHD patients and are classified as inflow, muscular, or perimembranous depending on their location in the septum. VSDs can also be iatrogenic from previous procedures including post-septal myomectomy or aortic valve replacement. Significant VSD can result in LV volume overload, ventricular failure and pulmonary hypertension, typically at a younger age than patients with ASDs. Therefore, most patients with large shunts are usually diagnosed and treated in childhood.
VSD closure in the adult is recommended for significant left-to-right shunt greater than 1.5:1, evidence of LV volume overload, symptoms of HF or when accompanied by progressive aortic regurgitation or after an episode of endocarditis.42 Device closure of VSDs has become more common and can be performed safely and effectively.43 Only muscular VSD closure is FDA approved. Since the introduction of the Amplatzer devices to close VSDs in 1998, the outcomes of percutaneous VSD closure have significantly improved and results have been promising as these were specifically designed for closure of VSDs.43 The wide variability in VSD location, size and morphology led to the development of different designs of the Amplatzer VSD devices (Figure 3). Other non-Amplatzers devices are also available for use. Additionally, perventricular ‘hybrid’ surgical approaches have been performed, in which a surgical incision exposes the right ventricular free wall followed by device closure in the usual fashion (Figure 8).
Patent Ductus Arteriosus
The overwhelming majority of patent ductus arterioses (PDA) are diagnosed and treated during childhood with medications or with catheter-based techniques. Our standard practice for PDA closure in the paediatric population is for hemodynamically significant shunt with evidence of left heart enlargement or when there is a continuous murmur. Silent PDA without an audible murmur is considered benign and does not warrant closure. PDA is a relatively uncommon finding in adults, usually discovered incidentally while investigating symptoms, such as dyspnoea or palpitations, evaluation of a murmur or after an episode of endarteritis. Similar to the paediatric population, large PDAs with significant left-to-right shunt should be closed to reduce occurrence of the sequelae of ventricular failure or pulmonary arterial hypertension.
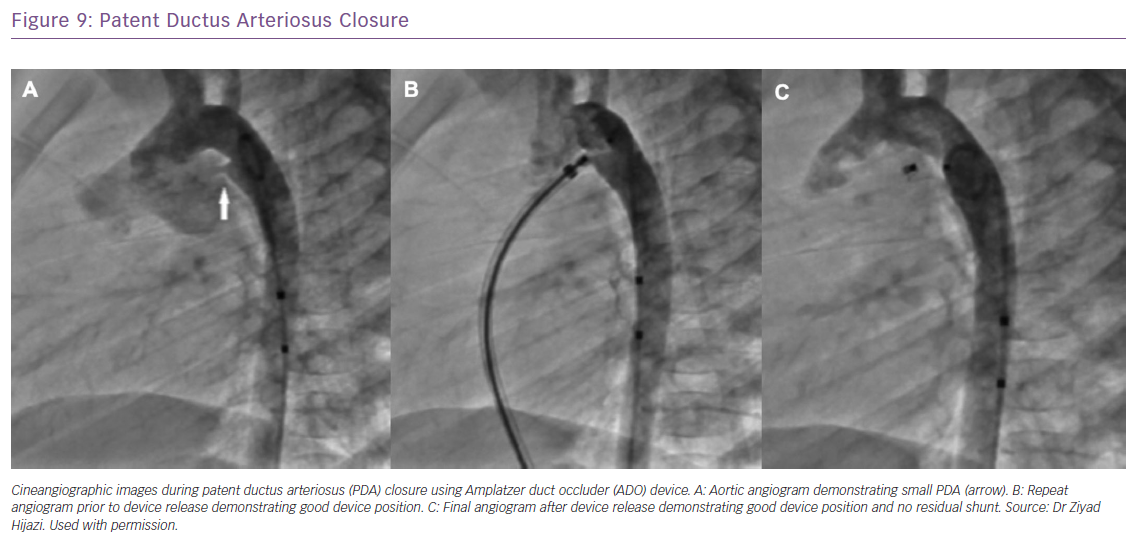
Catheter-based closure of PDAs in adults can be more challenging and complex compared with closure in the paediatric population, as PDAs tend to be calcified and more tortuous.44 Nevertheless, transcatheter closure remains more desirable compared with surgery because of the potential for recurrent laryngeal nerve damage during surgery for calcified PDAs. With the availability of different devices developed specifically for PDA closure, greater than 99% of PDAs are amenable to transcatheter closure (Figure 9).
Coronary Artery Fistula
Coronary artery fistulae are connections between the coronary arteries and the cardiac chambers or great vessels. The majority of fistulae originate from the right coronary artery, with the left anterior descending artery being the next most frequently involved. The major termination sites are the right cardiac chambers and pulmonary arteries. Less frequently, fistulae drain into the superior vena cava, coronary sinus or left cardiac chambers. Most fistulae are small and clinically silent. However, larger fistulae can lead to significant left-to-right shunting with right-sided volume overload and effective coronary arterial steal leading to ischaemia.
Most coronary fistulae are amenable to percutaneous closure either via a retrograde or antegrade approach, using a variety of coils, plugs or duct occluders. The aim of catheter closure is to occlude the fistulous artery as distally as possible, avoiding any possibility of occluding branches to the normal myocardium. Risks of fistula closure with these devices include MI (due to inadvertent closure of viable branches or retrograde propagation of thrombus occluding viable branches) and migration of coils or discs to extra-coronary vascular structures or in the coronary artery branches.45 Long-term effects of intravascular fistula occlusion are yet to be demonstrated.
Conclusion
As one of the leading causes of morbidity and mortality, HF has become a pandemic not only in the US but worldwide. Patients with SHD possess anatomical variations that may alter their response to medical therapy alone. Transcatheter-based structural interventions provide newer, less-invasive techniques accounting for anatomical variations that present a cornerstone of therapeutic options for these patients and the management of HF that can be expanded to the general population.