Heart failure (HF) affects more than 2% of the adult population worldwide; it is responsible for about 1 million hospitalisations per year in the US and it carries a high mortality risk.1 Recent population data indicate overall better survival rates, albeit with greater healthcare costs.1 Altogether, HF syndrome represents one of the biggest challenges to modern medicine, and places an enormous economic burden on society.
A large proportion of HF management costs comprise of ambulatory patient visits, emergency department visits and hospitalisations, with no difference between patients with HF with preserved and reduced ejection fraction (EF).1,2 Up to 40% of patients with HF have at least four hospitalisations during the course of the condition, and mortality risk increases with multiple previous hospitalisations.1,3 One of the contributory factors for repeat hospitalisations is low patient adherence to recommended drug treatments and lifestyle changes, shown to rapidly decline with the time elapsed from the previous hospitalisation.1–4
Advancements in HF drugs and devices, as well as in overall patient management and education, aim to improve outcomes, including a reduction in non-scheduled patient visits. Equally important, better understanding of underlying mechanisms of acute decompensated HF enables more timely management of patients at risk.5
Over the past decades and increasingly over the past several years, great effort has been invested in telemonitoring (TM) methods that would improve patient adherence, predict and/or prevent episodes of worsening HF and allow patients to be more closely monitored without presenting at healthcare centres. Over this time, various methods of remote patient care, monitoring and management have been introduced.
Definition and Types of Telemonitoring
TM or remote patient monitoring is a type of telemedicine. It is the provision of care to patients from care providers at a different location, using information technology. In general, telemedicine methods can be categorised to asynchronous (store and forward, non-simultaneous) or synchronous (real-time, simultaneous) depending on the timing of the information transfer.6,7 Both types have been used for TM of patients with chronic HF individually and in combination. As described in the existing literature, telemedicine for patients with HF mainly consists of the following:
- Structured telephone support (STS).
- Non-invasive TM of pre-specified parameters, such as daily weight, blood pressure, ECG, pulse oximetry, subjective assessment of HF symptoms or depression levels and medication adherence.
- Invasive TM by implanted devices with the sole function of remote patient monitoring (measuring surrogates of left ventricular filling pressures, such as right ventricular pressure, pulmonary artery pressure and left atrial pressure).
- Invasive TM by cardiovascular implantable electronic devices (CIEDs), such as ICDs or cardiac resynchronisation devices (CRT-D).8–11
Recommendations for Telemonitoring in Recent Heart Failure Guidelines
The European Society of Cardiology (ESC) provided limited recommendations for TM in its 2016 guidelines for the diagnosis and treatment of acute and chronic HF.12 Monitoring of pulmonary artery pressure (PAP) using a wireless implantable haemodynamic monitoring system (CardioMEMS HF System, Abbott) in symptomatic patients with reduced or preserved EF and a previous HF hospitalisation received a IIb class recommendation for the risk reduction of recurrent HF hospitalisations. The only other approach mentioned was multiparameter monitoring by ICD – an approach suggested by the Implant-based Multiparameter Telemonitoring of Patients with HF (IN-TIME) trial – that received the same recommendation for improvement of clinical outcomes in symptomatic patients with left ventricular EF (LVEF) ≤35%.12,13 All other TM methods were considered to lack sufficient evidence to support recommendation, based on different clinical trial results and lack of uniformity. An individual approach to the patient and TM method selection was highlighted.
The American College of Cardiology Foundation/American Heart Association Guidelines for the management of HF from 2013 did not provide any specific recommendation for TM practice, but stated that there was a need for clear evidence to identify the best processes of care.14 To fill that gap, the Heart Failure Society of America Scientific Statements Committee issued an official report on TM in 2018, highlighting the paucity of evidence from clinical trials to support the use of external electronic devices for TM (including STS and non-invasive TM), while implanted devices for monitoring PAP and/or other parameters may be beneficial in selected populations under a structured programme of care. The general message on TM was a need to shift the focus from using TM as a treatment to using it as a tool to improve organisation and effectiveness of care.15
The Canadian Cardiovascular Society gives no exact recommendation for TM and it is classified as an intervention with limited evidence of outcome improvement on a systematic level.16 On the other hand, the National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand HF Guidelines for the prevention, detection and management of HF recognise and have a strong GRADE recommendation for TM as a model of care to improve evidence-based practice in areas where access to a face-to-face multidisciplinary HF disease management programme after discharge is limited. TM of PAP by implantable devices received a weak GRADE strength of recommendation for patients with prior HF hospitalisation who are New York Heart Association (NYHA) class III, despite optimal care, with an aim to decrease hospitalisations for HF if a system is provided to ensure daily upload and weekly review of pressure data.17
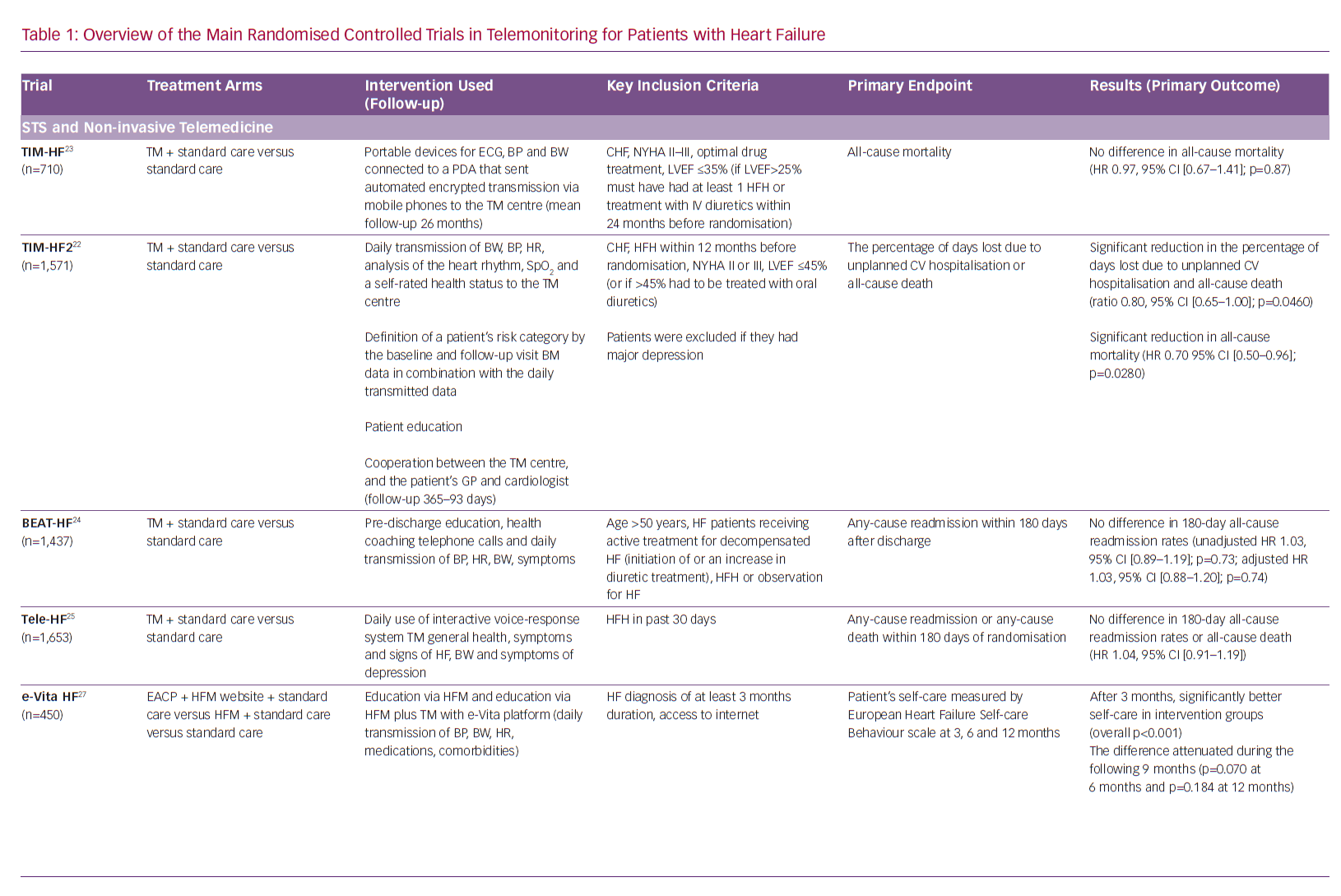
These recommendations by influential professional associations are driven by the results of randomised controlled trials (RCTs), some of which are large, but provide inconsistent results for TM care of HF patients (primarily STS and non-invasive TM; Table 1). Nonetheless, consecutive Cochrane reviews show a significant reduction in major outcomes (all-cause mortality and HF hospitalisations) when using STS or non-invasive TM, but these were not included in the latest ESC guidelines.18–20 In addition to the trials and meta-analyses, there is a growing number of expert opinion and review publications addressing the issue of equivocal evidence for the use of TM in the management of HF patients.8–11,21
Recent Evidence
Structured Telephone Support and Non-invasive Telemonitoring
The Efficacy of Telemedical Interventional Management in Patients with HF (TIM-HF2) RCT, conducted in multiple centres in Germany, included 1,571 patients with HF and a HF hospitalisation in the previous 12 months, NYHA functional class II or III and LVEF ≤45% or >45% if receiving a diuretic.22 Of note, exclusion criterion was major depression because the original TIM-HF trial had found that these patients did not respond well to TM.23 The primary outcome of TIM-HF2 was the percentage of days lost due to unplanned cardiovascular hospitalisation or all-cause death. The intervention included STS, daily remote transmission of multiple physical parameters to the 24/7 telemedical centre, assessment of patient’s risk for adverse events, education, and collaboration of the telemedical centre and patient’s healthcare providers (general practitioners and cardiologists). Patients in the intervention group had significantly lower percentage of days lost due to unplanned cardiovascular hospitalisation or death of any cause and significantly lower all-cause mortality, but not significantly lower cardiovascular mortality. The study showed that a specific multimodality TM is feasible and effective in terms of reduction of hospital days in a specific cohort of HF patients in the German healthcare system.22,23
Conversely, a multicentre RCT from California, US, Better Effectiveness After Transition – Heart Failure (BEAT-HF), studied 1,437 patients with treated HF with new or increased diuretics and recent HF hospitalisation, using pre-discharge HF education, regular health coaching telephone calls in combination with TM of multiple physical parameters as an intervention, and did not show a difference in readmission rates for any cause within 180 days after last discharge.24 However, BEAT-HF had a very low adherence rate to TM procedures, similar to the older Tele-HF trial (TM in patients with HF), another large RCT from the US that showed no significant differences in readmission rates for any reason, or death from any cause within 180 days in patients telemonitored by daily use of an interactive voice-response TM system.25 At most, 61.4% of patients were adherent to more than 50% of telephone calls and TM in the first 30 days in BEAT-HF, while in Tele-HF only 86% of patients randomised to the intervention used it at least once, and the adherence to intervention reduced to 55% by the final week of the study.24,25
Besides monitoring of physical or self-reported parameters, patient education on recognition of HF symptoms and signs, education on possible lifestyle changes to reduce the number of worsening HF episodes and surveillance of medication or diet adherence may be part of TM programmes aimed at improving self-care and self-management.26
A Dutch RCT, the EVIdence Based TreAtment – Heart Failure (e-Vita HF) study, has made an important addition to evidence for the value of patient education. It has shown improved short-term self-care of stable HF patients (NYHA class I and II) over 3 months by the intervention that promoted the use of the website heartfailurematters.org and/or an e-health adjusted care pathway using the e-Vita platform with TM equipment.27
Invasive Telemedicine with Implantable Devices for Remote Patient Monitoring
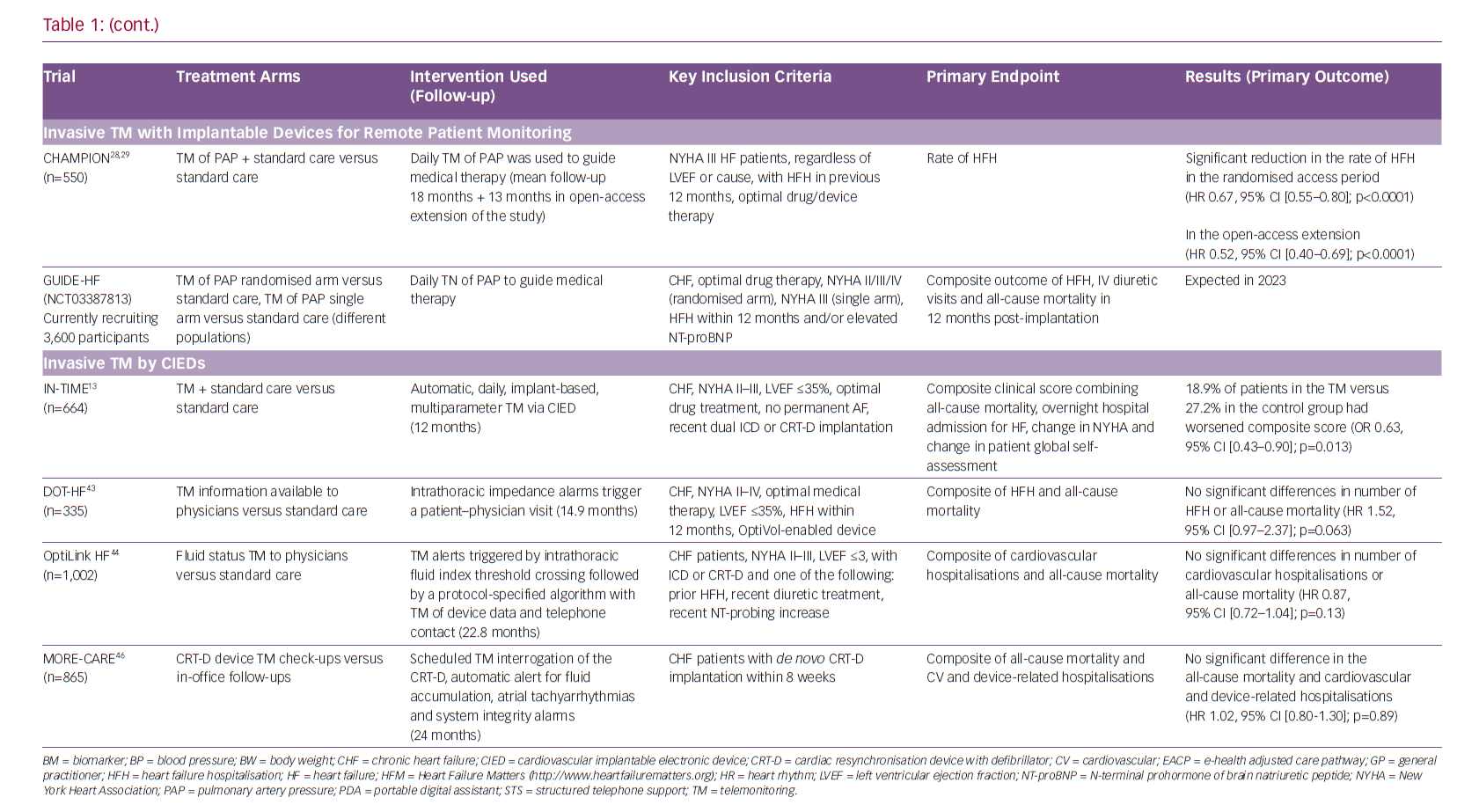
Recommendations by professional associations have included PAP monitoring by a specific device in a specific population, providing a supporting environment, but the level of recommendation is overall weak considering limited data from RCTs.12,14–17 The CardioMEMS implantable PAP sensor was proved to be effective in significantly reducing the rate of HF hospitalisations, decreasing PAP and improving quality of life of HF patients in the CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients (CHAMPION) RCT.28,29 The results supporting a reduction in HF hospitalisation for the intervention group were consistent across the initial phase of the RCT and during the continuous open-access period with overall 31 months of mean follow-up (HR 0.67, 95% CI [0.55–0.80]; p<0.0001 for the randomised controlled epoch and HR 0.52, 95% CI [0.40–0.69]; p<0.0001 for the open-access epoch).28,29 The obligatory post-approval study finished recruiting in 2018 and the preliminary results show that PAP-guided therapy of HF decreased PAPs, HF and all-cause hospitalisations in the real-world setting.30
Further studies done with this implantable PAP sensor were not RCTs, but they contributed data to the role of the PAP monitoring system in the management of HF patients. A retrospective cohort study on the use of haemodynamic TM in clinical practice included 1,114 patients with HF implanted with the device and showed a significant reduction in the number of HF hospitalisations after implantation; however, the study was observational and since it used Medicare claims data, little was known about patient characteristics and treatment prior to implantation.31, 32
Overall safety of the device in clinical practice was assessed as similar to the results of the CHAMPION trial (2.8% adverse events from 5,500 CardioMEMS HF System implants in the US versus 2.6% from 575 implant attempts in the CHAMPION trial), bearing in mind that the implantation is an invasive procedure with uncommon but possible serious injury to the pulmonary artery, that this resulted in deaths in the RCT and clinical practice.33
Cost-effectiveness of PAP monitoring has been studied for appropriate patient populations and deemed effective in studies in the US and the UK, although questions have arisen whether a TM system with additional cost-reduction benefits would become available.15,34,35
PAP monitoring has been shown to be useful in individual patients with advanced HF, while patients with left ventricular assist devices (LVAD) present another challenging patient population, in which continuous monitoring of PAP may improve proper timing and adequacy of LVAD optimisation.36 The Design and rationale of Haemodynamic guidance with CardioMEMS in patients with a left Ventricular Assist Device (HEMO-VAD) pilot study is planned to investigate the safety and feasibility of PAP monitoring for the optimisation of LVADs, while Investigation to Optimize Hemodynamic Management of Left Ventricular Assist Devices Using the CardioMEMS (Intellect 2; NCT03247829) is already under way.37
Further trials will produce data on the use of PAP monitoring in the broader HF population and these include the RCT Hemodynamic-GUIDEd Management of Heart Failure (GUIDE-HF; NCT03387813) and real-world setting trial CardioMEMS European Monitoring Study for Heart Failure (MEMS-HF).38
Several other PAP monitoring devices with different features are being researched, but there are no RCT data available so far. No new large RCT has been published with other implantable TM devices in recent years, of which left-atrial pressure monitoring devices should be mentioned, such as HeartPOD (Abbott) and V-LAP (Vectorious Medical Technologies).39 A niche for those devices exists since there is a substantial proportion of chronic HF patients where the estimation of PAP is not a real representation of left-sided filling pressures, such as patients with significant mitral valve disease, a component of pulmonary arterial hypertension or high pulmonary vascular resistance, pulmonary vasculature disease in chronic obstructive pulmonary disease or chronic thromboembolic disease.40 The primary concern lies in the implantation procedure that requires puncture of the interatrial septum with somewhat higher complication rates. The only prospective RCT of the HeartPOD system was terminated early due to a high rate of implant-related complications.39,41
Invasive Telemonitoring by Cardiovascular Implantable Electronic Devices
TM of HF patients with implanted devices seemed feasible and credible until it was tested in RCTs. Almost all trials with CIEDs in the role of early prediction of HF events – such as the Sensitivity and Positive Predictive Value of Implantable Intrathoracic Impedance Monitoring as a Predictor of Heart Failure Hospitalizations (SENSE-HF) trial, the Diagnostic Outcome Trial in Heart Failure (DOT-HF) trial and the Optimization of Heart Failure Management Using Medtronic OptiVol Fluid Status Monitoring and CareLink Network (OptiLink HF) trial – were neutral, and showed very low predictive value of measuring intrathoracic impedance – a surrogate of worsening fluid status or impending acute decompensation.42–44
TM of a single surrogate parameter of congestion, such as fluid index via a CIED was substituted with multiparameter TM, integrating several standard pacing parameters with symptoms and signs of HF and STS. This approach was successfully tested in the IN-TIME RCT, which showed how automatic CIED multiparameter monitoring can improved outcomes for HF patients. This evidence allowed the IN-TIME approach for TM to be included in the last ESC guidelines for the diagnosis and treatment of acute and chronic HF.12,13
A similar approach has been proven to reduce hospitalisation rates in the Telemonitoring in heart failure patients treated by CARdiac resynchronization Therapy with defibrillator (TELECART study, an Italian multicentre RCT that included 191 patients with an indication for CRT-D).45 Following CRT-D implantation, the patients were randomised to usual care versus usual care and intervention consisting of TM for ventricular and atrial tachyarrhythmias and premature contractions, low percentage of biventricular pacing, decreased patient activity and abnormal intracardiac electrograms.45 Similarly, a larger international multicentre RCT Monitoring Resynchronization devices and cardiac patients (MORE-CARE) showed significant reduction in healthcare resources in 865 CRT-D patients. Intervention in MORE-CARE included multiparameter TM of lung fluid accumulation, atrial arrhythmias and system integrity.46
A promising CIED diagnostic tool is the HeartLogic (Boston Scientific), a multisensor algorithm for CRT-D devices that was tested in an international, multicentre study Multisensor Chronic Evaluation in Ambulatory Heart Failure Patients (MultiSENSE).47 The HeartLogic index consists of sensing heart sounds, thoracic impedance, respiration rate and its ratio to tidal volume, heart rate and patient activity. In the MultiSENSE study, the sensitivity for detection of HF events was up to 70%, while the median time from the alert onset to the event occurrence was 34 days, indicating the potential for a very early warning of worsening HF. Although further observational studies with this diagnostic tool are ongoing, RCTs are lacking.
Besides CIEDs, LVADs may also demand optimisations that require healthcare resources and generate numerous parameters that could potentially be telemonitored. The most frequently used LVADs (HeartMate II or HeartMate 3 [Abbott] and Heart Ware HVAD [Medtronic] currently do not have options for TM, posing a challenge for their future development. Currently available VADs capable of TM are the HeartAssist 5 LVAD and aVAD (ReliantHeart). The first experience with remote monitoring of LVAD patients has been recently published, emphasising its importance in early detection of pump thrombosis, oscillations of volume status or arrhythmias.48
Conclusion
Overall, TM for HF is still scarcely represented in the recommendations from professional associations, except for PAP monitoring, which is supported by RCT data. The paucity of evidence required to base informed recommendations may seem surprising, especially considering the current wide availability of different e-health technologies and the increase in recent popularity of health devices. This reality is a result of the enormous heterogeneity of TM devices tested of differences in selected patient populations, such as type of HF, age, LVEF, clinical stage, background treatment of geographical determinants (densely populated against remote areas far from HF centres) and variabilities between healthcare systems. Furthermore, the strengthening of regulatory processes over time provides additional variability in the testing and approval of TM devices. All these factors contribute to the body of evidence that provides arguments both for and against different types of TM for HF.